
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Characteristics of the Immunoglobulin (Ig) Classes
|
|
|
|
The Classes of Immunoglobulins
Accessory Molecules on Immunoglobulins
Functions of the Crystallizable Fragment
FIGURE 3 Summary of antibody functions.
Although the Fab fragments bind antigen, the Fc fragment has a different binding function. In most classes of immunoglobulin, the proximal end of Fc contains an effector molecule that can bind to certain receptors on the membrane of cells, such as macrophages, neutrophils, eosinophils, mast cells, basophils, and lymphocytes. The effect on an antibody’s Fc fragment binding to a cell receptor depends upon that cell’s role. In the case of opsonization, the attachment of antibody to foreign cells and viruses exposes the Fc fragments to phagocytes. Certain antibodies have receptors on the Fc portion for fixing complement, and in some immune reactions, the binding of Fc causes the release of cytokines. For example, the antibody of allergy (IgE) binds to basophils and mast cells, which causes the release of allergic mediators such as histamine. The size and amino acid composition of Fc also determine an antibody’s permeability, its distribution in the body, and its class.
All antibodies contain molecules in addition to the basic polypeptides. Varying amounts of carbohydrates are affixed to the constant regions in most instances (table 1). Two additional accessory molecules are the J chain that joins the monomers of IgA and IgM, and the secretory component, which helps move Ig across mucous membranes. These proteins occur only in certain immunoglobulin classes.
Immunoglobulins exist as structural and functional classes called isotypes (compared and contrasted in table 1). The differences in these classes are due primarily to variations in the Fc fragment and its accessory molecules. The classes are differentiated with shorthand names (Ig, followed by a letter: IgG, IgA, IgM, IgD, IgE).
The structure of IgG has already been presented. It is a monomer produced by memory cells responding the second time to a given antigenic stimulus. It is by far the most prevalent antibody circulating throughout the tissue fluids and blood. It has numerous functions: It neutralizes toxins, opsonizes, and fixes complement, and it is the only antibody capable of crossing the placenta.
Table 1
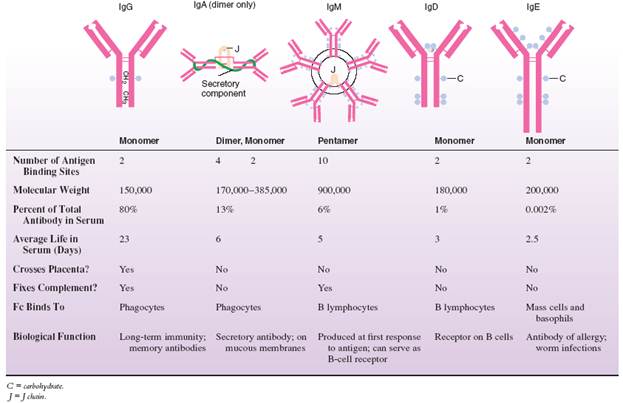
The two forms of IgA are: (1) a monomer that circulates in small amounts in the blood and (2) a dimer that is a significant component of the mucous and serous secretions of the salivary glands, intestine, nasal membrane, breast, lung, and genitourinary tract. The dimer, called secretory IgA, is formed in a plasma cell by two monomers attached by a J piece. To facilitate the transport of IgA across membranes, a secretory piece is later added by the gland cells themselves. IgA coats the surface of these membranes and appears free in saliva, tears, and mucus. It confers the most important specific local immunity to enteric, respiratory, and genitourinary pathogens. It protects newborns who derive it passively from nursing.
|
|
|
IgM (M for macro) is a huge molecule composed of five monomers (making it a pentamer) attached by the Fc receptors to a central J chain. With its 10 binding sites, this molecule has tremendous avidity for antigen (avidity means the capacity to bind antigens). It is the first class synthesized by a plasma cell following its first encounter with antigen. Its complement-fixing and opsonizing qualities make it an important antibody in many immune reactions. It circulates mainly in the blood and is far too large to cross the placental barrier.
IgD is a monomer found in miniscule amounts in the serum, and it does not fix complement, opsonize, or cross the placenta. Its main function is to serve as a receptor for antigen on B cells, usually along with IgM. It seems to be the triggering molecule for B-cell activation, and it can also play a role in immune suppression.
IgE is also an uncommon blood component unless one is allergic or has a parasitic worm infection. Its Fc region interacts with receptors on mast cells and basophils. Its biological significance is to stimulate an inflammatory response through the release of potent physiological substances by the basophils and mast cells. Because inflammation would enlist blood cells such as eosinophils and lymphocytes to the site of infection, it would certainly be one defense against parasites. Unfortunately, IgE has another, more insidious effect – that of mediating anaphylaxis, asthma, and certain other allergies.
6. Monoclonal Antibodies: Useful Products from Cancer Cells
For many years, antiserum extracted from human or animal blood was the main source of antibodies for tests and therapy, but most antiserum has a basic problem. It contains polyclonal antibodies, meaning that it is a mixture of different antibodies because it reflects dozens of immune reactions from a wide variety of B-cell clones. This characteristic is to be expected, because several immune reactions may be occurring simultaneously, and even a single species of microbe can stimulate several different types of antibodies. Certain applications in immunology require a pure preparation of monoclonal antibodies (MABs) that originate from a single clone and have a single specificity for antigen.
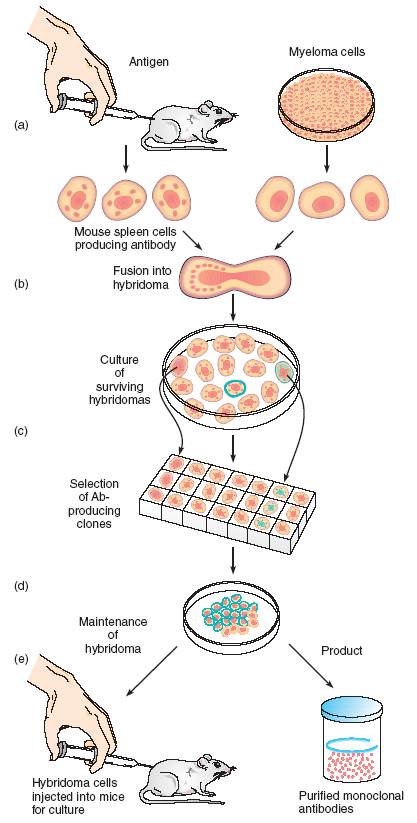
The technology for producing monoclonal antibodies is possible by hybridizing cancer cells and plasma cells in vitro (figure 4). This technique began with the discovery that tumors isolated from multiple myelomas in mice consist of identical plasma cells. These monoclonal plasma cells secrete a strikingly pure form of antibodies with a single specificity and continue to divide indefinitely. Immunologists recognized the potential in these plasma cells and devised a hybridoma approach to creating MABs. The basic idea behind this approach is to hybridize or fuse a myeloma cell with a normal plasma cell from a mouse spleen to create an immortal cell that secretes a supply of functional antibodies with a single specificity.
Laboratories use MABs to identify antigens, receptors, and antibodies; to differentiate cell types (T cells versus B cells) and cell subtypes (different sets of T cells); to diagnose diseases such as cancer and AIDS; and to identify bacteria and viruses.
A number of promising techniques have been directed toward using monoclonals as drugs. When genetic engineering is combined with hybridoma technology, the potential for producing antibodies of almost any desired specificity and makeup is possible. For instance, chimeric MABs (monoclonal antibodies) that are part human and part mouse have been produced through splicing antibody genes. It is even possible to design an antibody molecule that has antigen binding sites with different specificities. Monoclonals can be hybridized with plant or bacterial toxins to form immunotoxin complexes that attach to a target cell and poison (Figure 5). The most exciting prospect of this therapy is that it can destroy a specified cancer cell and not harm normal cells. Monoclonals are currently employed in treatment of cancers such as breast and colon cancers. Drugs such as Rituxan and herceptin bind to cancer cells and trigger their death. Such antibodies could also be used to suppress allergies, autoimmunities, and graft rejection. Drugs such as OKT3 and Orthoclone are currently used to prevent the rejection of organ transplants by incapacitating cytotoxic T cells. Now that researchers have genetically engineered plants and mice to produce human MABs, therapeutic uses will continue to expand. MABs are undergoing clinical trials to treat heart, lung, and inflammatory diseases.
|
|
|
|
|
|
|
Дата добавления: 2014-01-11; Просмотров: 5216; Нарушение авторских прав?; Мы поможем в написании вашей работы!