
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Methodical guidance for laboratory work №14
|
|
|
|
Methodical recommendation for the laboratory lesson
SEMEY STATE MEDICAL ACADEMY
Specialty: 0501301General medicine
Discipline: chemistry
Chair: of chemistry and technology of medicine
Higher base medical education, course – 1
Topic №14: Olygo- and polysaccharides.
Semey 2007
Approved
on the chair meeting from “_____”____________2007.
Protocol № ____
Head of the chair of chemistry and technology of medicine,
prof. __________ Gavrilenko I.V
1.Topic№14: Olygo- and homopolysaccharides.
2.Aim: to study the structure and properties of disaccharides, classification and properties of homopolysaccharides and its biological significance.
3.Objectives of teaching: Fulfil the qualitative reactions on disaccharides, conduct acid hydrolysis of the starch. Study of topic promotes forming in student of knowledge about structure and properties of disaccharides and polysaccharides, as the base for metabolic processes understanding.
4. Basic questions:
1. Concept about complex carbohydrates, its classification.
2. Disaccharides, structure, composition, properties
a) reduced disaccharides (maltose, cellibiose, lactose)
b) unreduced disaccharides
3. Homopolysaccharides, structure, composition, properties
а) starch, hydrolysis of starch
b) glycogen, energetic function
c) decstrane, as substitutes of plasma blood
d) cellobiose, ester’s derivatives of cellobiose
5. Methods of teaching:
The determining initial level knowledge’s of student on chemistry. The conversation and questioning on topic of the lesson. Fulfilling the laboratory work and protection of the report.
Experiment №1. Studying of disacclmride reduction properties.
Pour in three 5-6drops 1% solution of sacarose, maltose and lactose. Then fulfill with all of the t-t Pheling’s reaction and indicate the what disaccharide reduce copper hydoxide.
Table 1.
| Disaccharides | Pheling’s reaction | Selivanof reaction | Reduced and unreduced disaccharides |
| maltose | |||
| lactose | |||
| sacarose |







 Experiment№2. Selivanof reaction with disaccharide:Pour in the 3 test-tubes 5-6 drops 1% solutions of sacarose, maltose, lactose and fulfill the same reaction butwithSelivanofreagent.Comparethe results and make a conclusion in what disaccharide fructose presents.Write the results int o the table. Positive result indicate as (+) and negative as (-). Make a conclusion.
Experiment№2. Selivanof reaction with disaccharide:Pour in the 3 test-tubes 5-6 drops 1% solutions of sacarose, maltose, lactose and fulfill the same reaction butwithSelivanofreagent.Comparethe results and make a conclusion in what disaccharide fructose presents.Write the results int o the table. Positive result indicate as (+) and negative as (-). Make a conclusion.
Experiment№3. Acidic hydrolysis (starch dextinisation)
Pour the t-t 3 ml 1% solution of starchand add l ml 10% solution of sulfuricacid and put the mixture into the water andeach 2-3 minmake the analysis with Lugol reagent and write the colors into the table. Make the analysis till liquid remains without color change. Then make the Pheling’s reaction and watch the formation of red p.p.t. of copper oxide(I)
|
|
|
Table 2.
| № tests | |||||||||||
| Color of liquids | |||||||||||
| Name dextrine |
During the hydrolysisthe starch is destroyed until intermediate products. The scheme of this destruction is:
(С6Н10О5)n + (n-x)Н2O → (С6Н10О5)m + xН2O → nС6Н12O6, where n>m
Starch with iodine gives blue color
¯
Amilodextrines – with iodine gives from blue-violet before dark-violet color
¯
Phlavodextrines - with iodine gives yellow-orange color
¯
Eritrodextrines - with iodine gives from russet before is dyed- orange
¯
 Ahrodextrines
Ahrodextrines
¯
Maltodextrines
¯ do not give any color with iodine
Maltose
¯
Glucose
Thelast 4 compounds do not give any color with iodine.
6. Literature:
1. J. M. Sehgal. Modern Chemistry, class XI, Delhi, 1989 - 15 items.
2. Jean B. Umland & John M. Bellama, General Chemistry. Houston. USA. 1996 - 1 item.
3. Darrel D. Ebbing, General Chemistry, Wayne University, USA. 1990 - 3 items.
4. Karen C. Timberlake, Chemistry - An Introduction to General. Organic and Biochemistry, Los Angeles, USA, 1991-1 item.
7.Control:
Fulfill the exercises:
1. Write structure of the lactose by formula of Cheourse
2. What disaccharides are a structure unit of amilose.
3. Name of components, being included in gialuronic acid.
4. Name of components being included in chondrotionsulphane. Indicate the types of bond between monosaccharide link this heteropolysaccharide.
5.Write structure of maltose by formula of Cheourse
6. What monosaccharide is a structure unit of cellulose?
7. Define, formulas what disaccharides:
 ;
; 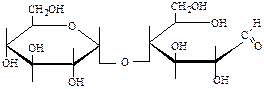
|
|
|
|
Дата добавления: 2015-07-13; Просмотров: 539; Нарушение авторских прав?; Мы поможем в написании вашей работы!