
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Surface in active substances
|
|
|
|
Surface active substances. Rule of Traube-Duclo.
Sorption and its kinds
Absorption is a physical or chemical phenomenon or a process in which atoms, molecules, or ions enter some bulk phase – gas, liquid, or solid material. This is a different process from adsorption, since molecules undergoing absorption are taken up by the volume, not by the surface (as in the case for adsorption). A more general term is sorption, which covers absorption, adsorption, and ion exchange. Absorption is a condition in which something takes in another substance
Adsorption is the adhesion of atoms, ions, or molecules from a gas, liquid, or dissolved solid to a surface.This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid (the absorbate) permeates or is dissolved by a liquid or solid (the absorbent). Note that adsorption is a surface-based process while absorption involves the whole volume of the material. The term sorption encompasses both processes, while desorption is the reverse of adsorption. It is a surface phenomenon.
Ion exchange is an exchange of ions between two electrolytes or between an electrolyte solution and a complex. In most cases the term is used to denote the processes of purification, separation, and decontamination of aqueous and other ion-containing solutions with solid polymeric or mineralic 'ion exchangers'.
Surface active substances, also known as surfactants, are those substances which preferentially adsorb at the air-liquid, liquid-liquid or liquid-solid interfaces. The surface activity of a solute refers to a particular solvent. Molecules of surface active substances contain at least two distinct parts, a moiety which interacts strongly with the solvent, the lyophilic part, and another moiety the lyophobic part, whose interaction with the solvent is less than its interaction with molecules of a structure similar to its own.
Inactive substances which increase the surface tension of the liquid in which they are dissolved, may induce capillary wave instability at the surface of the solution.
It is shown that the simultaneous the free surface of a solution of an inactive substance in the concentration.
5. Gibb’s equation. Gibb’s isotherm of surface tension.
The Gibbs adsorption isotherm for multicomponent systems is an equation used to relate the changes in concentration of a component in contact with a surface with changes in the surface tension. For a binary system containing two components, the Gibbs adsorption equation in terms of surface excess is:

where
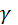 is the surface tension,
is the surface tension,
Г1 is the surface excess of component 1,
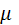 is the chemical potential of component 1.
is the chemical potential of component 1.
|
|
|
|
|
Дата добавления: 2014-12-08; Просмотров: 2299; Нарушение авторских прав?; Мы поможем в написании вашей работы!