
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Reading. How semiconductors work
|
|
|
|
Start here
HOW SEMICONDUCTORS WORK
Speaking
7. Work in pairs. Discuss the difference between the types of semiconductors, agreeing or disagreeing, rather than just making short statements. Use the correct forms of the verb “to be”.
1. Work in small groups. Study the electron orbital zone in Fig.1.2 and discuss these questions.
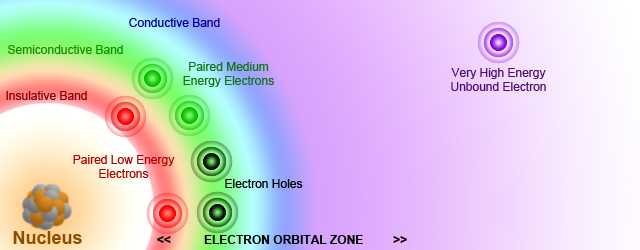
Fig. 1.2. Electron orbital zone.
1) Does the information depicted in the diagram have any connection with our previous lessons?
2) How do you think it works?
2. With your group, read the text, divide it into parts and entitle them. Come up with the headline. Answer the questions below.
To understand how semiconductors work, you must first understand a little about how electrons are organized in an atom. The electrons in an isolated atom are organized in energy levels. The outermost level is called the valence level. The electrons in this level are the ones, known as valence electrons that form bonds with neighboring atoms. Such bonds are called covalent bonds. If all the neighboring atoms are of the same type, it's possible for all the valence electrons to bind with valence electrons from other atoms. When that happens, the atoms arrange themselves into structures called crystals. Semiconductors are made out of such crystals, usually silicon crystals.
Atoms within a crystal have a marked effect upon each other. The forces that bind these atoms together greatly modify the behavior of the other electrons. One consequence of this close proximity of atoms is to cause the individual energy levels of an atom to break up and form bands of energy. If for isolated atoms we have energy levels, for big spread molecules, macromolecules, and also crystals we have energy bands.
The energy band formed by a series of energy levels containing valence electrons is known as valence band. Electrons in this band are more tightly bound to the individual atom than the electrons in the conduction band. However, the electrons in the valence band can still be moved to the conduction band with the application of energy, usually thermal energy.
The upper band in the solid is called the conduction band because electrons in this band are easily removed by the application of external electric fields. These electrons are free enough to move and thereby carry an electric current.
The energy difference between a valence band and a conduction band is called the forbidden band. Electrons are never found in this band, but may travel back and forth through it. Technically, the band gap is the energy it takes to move electrons from the valence band to the conduction band. The width of the forbidden band determines whether a substance is an insulator, semiconductor, or conductor.
Questions:
1) How are electrons in an isolated atom organized?
2) How is the outermost energy level in an isolated atom called?
3) What electrons form bonds with neighboring atoms? How are such bonds called?
4) When do atoms arrange themselves into crystals?
5) Are semiconductors made out of isolated atoms?
6) How are atoms arranged in crystals and molecules?
7) What is the difference between a valence band and a conduction band?
8) What is a forbidden band?
|
|
|
|
|
Дата добавления: 2014-11-29; Просмотров: 429; Нарушение авторских прав?; Мы поможем в написании вашей работы!