
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Specify sulfate - ion
|
|
|
|
Specify the chloride-ion
Specify metal with valence 3
The charge of the proton is equal to
А) +1
А) Al
А) Cl-
А) SO2-4
295. To define coordination numbers of forming complexes in compounds: K  ,
, 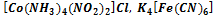
A) 2, 6, 6
296. To define a charge of forming complex in compound 
A) +2, +3
297. What is the type of reaction H2SO4+ 2NaOH =Na2SO4+ 2Н2O:
I) neutralization; 2) replacement; 3) decomposition; 4) addition; 5) oxidation-reduction?
A) 1
298. What reactions belong to the oxidation-reduction?

2.  ;
;
3.  ;
;
4. Fe+S=FeS
A) 2, 4
299. What is the type of reaction:  ?
?
A) Disproportion
300. Oxidizer:
Accepts electrons;
301. Reducer:
A) Gives electrons;
302. Oxidizer for the given reaction: 
A) 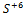 ,
,
303. Reducer for the given reaction: 4NH3+3O2=2N2+6H2O
A. N-3
304. To specify the sum of coefficients for reaction: 
A) 10
305. To specify the sum of coefficients for reaction: 
А)20
306. To specify the sum of coefficients for reaction: K2S +K2Cr2  +H2SО4
+H2SО4  S + K2S04 + Cr2 (SО4
S + K2S04 + Cr2 (SО4  +
+ 
А) 26
307. To specify the sum of coefficients for reaction:  +
+ 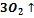
A.7
308. To specify the sum of coefficients for reaction: 
A) 13
309. To specify the sum of coefficients for reaction: 
A) 19
310. To specify the sum of coefficients for reaction: 
A) 7
311. To specify the sum of coefficients for reaction: 
A) 22
312. To specify the sum of coefficients for reaction:  →
→ 

A) 10
313. To specify the sum of coefficients for reaction:  +
+  +
+  →Cr
→Cr  +
+  +
+  O
O
A) 14
314. pH of solution calculates according to the formula:
A) pH = 14 - pOH
315. The hydroxyl indicator of environment is equal:
A) pH = [H+][OH-]
316. To specify an alkaline solution:
A) [H+] = 10-12
317. To specify a neutral solution:
A) H+] = [OH-]
318. Electrolysis is:
A) oxidation-reduction process;
319. Cathode is an electrode at which there is a process:
A) Reduction of cations;
320. Anode is an electrode at which there is a process:
A) Oxidation of anions;
321. E lectrolyte of the lead accumulator is the solution of:
A) H2SO4
322. Inhibitor is:
diminish of corrosion
323. Faraday number is equal:
A) 96500
324. Potential of a standard hydrogen electrode is equal:
A) 0
325. To inert anodes belong:
A) graphite, platinum
326. To active anodes belong:
A) copper
327. At electrolysis of NaCl solution at the anode it is allocated:
A) chlorine
|
|
|
|
|
Дата добавления: 2017-01-13; Просмотров: 264; Нарушение авторских прав?; Мы поможем в написании вашей работы!