
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Chemical accident
|
|
|
|
Strong toxic substance is the primary hazard of chemical accident. The most widely used strong toxic substances are chlorine, ammonia, sulfuric anhydride, hydrogen sulfide, hydrocyanic acid, benzol, mercury.
Chlorine (Cl2): green-yellow gas with strong smell. Boiling point – 34.6 deg C. Peack concentration 0.1 mg/l is fatal.
Technologic use: producing hydrochloric acid, bleaching, sterilization of potable water and deactivation of wastes.
Health effects: inflammation of tissues where they are deposited. It may cause reactions like eczema or dermatitis, shortness of breath, inflammatory responses and oedema.
Ammonia (NH3): colorless gas with strong smell. It becomes liquid at temperature -33.4 deg C, solid – at -77.8 deg C. 30-minutes exposure to concentration 7 mg/l is fatal. It becomes liquid at relatively low pressure 0.7-0.8 MPa.
Since ammonia vaporizing absorbs essential amount of heat it is used in cooling systems.
Health effects: cramps and inflammation of lungs and larynx which can be fatal. Skin burns.
Sulfuric anhydride (SO2): colorless gas with strong smell. It becomes liquid at temperature -10 deg C, solid – at -75 deg C. Twice heavier than air. Reaction with air gives sulfuric acid. It’s produced by burning sulfur.
Technologic use: production of sulfuric acid, bleaching in papermaking and fabric making, conservation in food industry, disinfection.
Health effects: coughing, eyes pain, difficult respiring and swallowing, skin inflammation.
Hydrogen sulfide (H2S): colorless gas with strong smell. It becomes liquid at temperature -60.3 deg C, solid – at -85.5 deg C. 4-45% airborne concentration is explosive. It’s produced as secondary product in petroleum or natural gas refining.
Technologic use: producing sulfuric acid, sulfur.
Medical use: sulfurated hydrogen baths (revitalizing health effect).
Health effects: irritating respiration organs, skin and mucous membranes.
Hydrocyanic acid (HCN): colorless light liquid, has almond fragrance. Health effects: loss of consciousness, paralysis of respiratory tract.
Benzol (C6H6): colorless liquid, its vapor is heavier than air and explosive. Health effects: weakness, headache, dizziness, sickness, muscle cramp.
Mercury (Hg): metal that is liquid at room temperature. Its vapor is poisonous. Mercuric chloride used in dressing skin is very toxic.
To assess risk associated with chemical accident one should consider exposure, dose and response.
Toxicity is the intrinsic capacity of a chemical agent to affect an organism adversely.
Xenobiotics is a term for “foreign substances”, that is, foreign to the organism. Its opposite is endogenous compounds. Xenobiotics include drugs, industrial chemicals, naturally occurring poisons and environmental pollutants.
Hazard is the potential for the toxicity to be realized in a specific setting or situation.
A dose is often expressed as the amount of a xenobiotic entering an organism (in units such as mg/kg body weight). The dose may be expressed in different (more or less informative) ways:
1. exposure dose, which is the air concentration of pollutant inhaled during a certain time period (in work hygiene usually eight hours),
2. or the retained or absorbed dose (in industrial hygiene also called the body burden), which is the amount present in the body at a certain time during or after exposure.
3. The tissue dose is the amount of substance in a specific tissue
4. and the target dose is the amount of substance (usually a metabolite) bound to the critical molecule. The target dose can be expressed as mg chemical bound per mg of a specific macromolecule in the tissue.
To apply this concept, information on the mechanism of toxic action on the molecular level is needed. The target dose is more exactly associated with the toxic effect. The exposure dose or body burden may be more easily available, but these are less precisely related to the effect.
In the dose concept a time aspect is often included, even if it is not always expressed. The theoretical dose according to Haber's law is
D = CT, (21)
where D is dose, C is concentration of the xenobiotic in the air and T the duration of exposure to the chemical. If this concept is used at the target organ or molecular level, the amount per mg tissue or molecule over a certain time may be used. The time aspect is usually more important for understanding repeated exposures and chronic effects than for single exposures and acute effects.
Latency time is the time between first exposure and the appearance of a detectable effect or response. The term is often used for carcinogenic effects, where tumours may appear a long time after the start of exposure and sometimes long after the cessation of exposure.
A dose threshold is a dose level below which no observable effect occurs. Thresholds are thought to exist for certain effects, like acute toxic effects; but not for others, like carcinogenic effects (by DNA-adduct-forming initiators). The mere absence of a response in a given population should not, however, be taken as evidence for the existence of a threshold. Absence of response could be due to simple statistical phenomena: an adverse effect occurring at low frequency may not be detectable in a small population.
LD50 (lethal dose) is the dose causing 50% lethality in an animal population. The 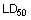 is often given in older literature as a measure of acute toxicity of chemicals. The higher the
is often given in older literature as a measure of acute toxicity of chemicals. The higher the 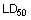 , the lower is the acute toxicity. A highly toxic chemical (with a low
, the lower is the acute toxicity. A highly toxic chemical (with a low 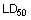 ) is said to be potent. There is no necessary correlation between acute and chronic toxicity.
) is said to be potent. There is no necessary correlation between acute and chronic toxicity.
ED50 (effective dose) is the dose causing a specific effect other than lethality in 50% of the animals.
Chemical accident is breakdown of technological process, damage of pipelines, tanks, reservoirs, transport facilities that causes ejection into environment of strong toxic substances.
Zone of chemical pollution includes spot (I) of ejected chemical agents and territory polluted with their vapor cloud (II) (fig. 9).
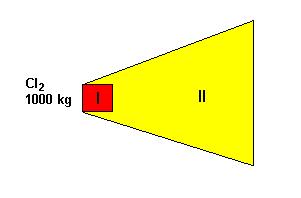
Fig. 9 Zone of chemical pollution
Alarm signal about chemical accident “Attention!”, sirens or interrupting horn sound and also instructions about how to protect houses, food and water are broadcasted via loudspeakers.
If you hear that sound, turn on TV and Radio to get more information about accident and instruction how to behave. Shut the windows, shut down electricity and turn off the gas. Put on rubber boots, coat, take documents, wear, 3-day storable food, inform your neighbors and quickly leave the house. Leave possible zone of chemical pollution moving perpendicular to wind. Go the distance about 1.5 km from your current position. To protect respiratory organs wear anti-gas, filter mask, or just wet fabric. It’s recommended to wet fabric with 2-5% baking soda mixture to protect against the chlorine or 2% lemon or acetic acid to protect against ammonia. If you can’t leave polluted zone, shut the doors, widows, ventilation and chimney tight. Seal the gaps in them with scotch or paper. Don’t hide in basements or at the first floor. If you suspect chemical poisoning try to avoid physical exertion, drink much milk, tea and immediately go to doctor.
|
|
|
|
Дата добавления: 2014-01-04; Просмотров: 402; Нарушение авторских прав?; Мы поможем в написании вашей работы!