
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Alkynes alkadienes alkenes
|
|
|
|
С п Н2 п С п Н2 п -2 С п Н2 п -2
Figure 1. Classification of organic substances according to the structure of carbon skeleton.
Classes of hydrocarbon derivatives on the presence of functional groups are the following:
- halogen derivatives R-Hal: CH3CH2Cl (clorinethane), C6H5Br (bromebensole);
- alcohols and phenols R-OH: CH3CH2OH (ethanol), C6H5OH (phenol);
- tioles R-SH: CH3CH2SH (ethanetiole), C6H5SH (tiophenol);
- 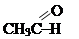 simple ethers R-O-R: CH3CH2-O-CH2CH3 (diethyl ether), complex ethers R-CO-O-R: CH3CH2COOCH2CH3 (ethyl ether of acetic acid);
simple ethers R-O-R: CH3CH2-O-CH2CH3 (diethyl ether), complex ethers R-CO-O-R: CH3CH2COOCH2CH3 (ethyl ether of acetic acid);
- 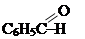 carbonile combinations: aldehydes R-CHO: (ethanal),
carbonile combinations: aldehydes R-CHO: (ethanal),

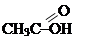 (bensaldehyde), ketones R-CO-R: CH3COCH3 (propanol), C6H5COCH3 (methylphenilketone);
(bensaldehyde), ketones R-CO-R: CH3COCH3 (propanol), C6H5COCH3 (methylphenilketone);
- carboxylic acids RCOOH: (acetic acid), (bensoin acid);
- sulpho acids R-SO3H: CH3SO3H (methanesulphoacid), C6H5SO3H (bensolsulphoacid);
- amines R-NH2: CH3CH2NH2 (ethylamine), CH3NHCH3 (dimethylamine), C6H5NH2 (aniline);
- nitro connections R-NO2: CH3CH2NO2 (nitroethane), C6H5NO2 (nitrobensol);
- metal organic (element organic) combinations: CH3CH2Na (ethylnitrium).
Rank of similar structured combinations, having similar chemical features, in which separate members of the rank differ from each other only by the number of –CH2- groups, are called homologic rank, and –CH2- group is called homologic diversity.
Reactions with the majority of members of homologic rank go in the same way (the exception are also the first members of the rank). Thus, if you know chemical reactions of only one member of the rank, you can state with a high degree of possibility, that the similar transformations take place with the rest members of the homologic rank.
A general formula can be defined for any homologic rank, which reflects the relation between carbon and hydrogen atoms at members of this rank. Such formula is called general formula of the homologic rank. Thus, CnH2n+2 – formula of alkynes, CnH2n+1OH – is a formula of alyphatic single atom alcohols.
Nomenclature of organic combinations could be trivial, rational and systematic nomenclature. Trivial nomenclature is a unity of historical names. Thus, from the name we could judge where was apple acid received or lemon acid, what is the way in which pirogrape acid was produced (it is pirolysis of grape acid). Rational nomenclature build the names on the basis of the structure of a more simple combination (the first member of the homologic rank). CH3OH – carbinol then, CH3CH2OH – methylcarbinol, CH3CH(OH)CH3 – dimethylcarbinol and so on.
Nomenclature IUPAC (international union of practical and applied chemistry) is called a systematic nomenclature. The names of hydrocarbons and their derivatives is based on the name of corresponding hydrocarbon with addition prefixes and suffixes belonging to this homologic rank. See information to the first seminar.
1.4. Electron structure of carbon atom, hybridization.
For the valent electron layer of carbon atom C, which is in the main subgroup of the fourth group of the second period in Mendeleev’s Periodical table, the main qwant number n=2, collateral (orbital) qwant number l = 0 (s-orbital) and 1 (p-orbital), magnet qwant number m=0 (at l=0) and –1,0,1 (at l=1).
In excited state on the outer electron layer of carbon atom there is one electron on one s-orbital and three p-orbital. Alkanes are characterized by sp3-hybridization (all 4 atom orbitals of the outer electron layer participate). In non-bounding combinations 1 or 2 non-hybridized p-orbitals take part in formation of π-bonds, and the type of hybridization of carbon atom is –sp3 for alkynes and –sp for alkenes.
Hybrid orbitals of alkanes are placed symmetrically in the space and are directed to the tops of a tetraedre. C-H bond is formed by overlap s-orbital of H atom by hybrid orbital of C atom, C-C bond is formed by overlap of two hybrid orbitals (the direction of the bond is along the axis between atoms). This is s-bond.
Features of s-bond:
- relative chemical inertion due to high solidity;
- maximum of electron density is placed symmetrically relative to the axis, connecting atoms, that is why a free rotation is possible along this axis without changes in orbital overlap (conformers);
- the length of the bond is 0,154 nm; the corner between orbital directions is 109,5°;
- electronegativity of C atom in sp3-hybrid state is 2,51.
Carbon atom connected by a double bond to another carbon atom, is in the state of sp2-hybridization (3 atom orbitals of the outer electron layer participate). Hybrid orbitals are placed in the space symmetrically in one plain, containing carbon nuclei. The rest non-hybrid p-atom orbital is oriented perpendicularly to this plain. C-H bond forms by the overlap of s-orbital of H atom and hybrid orbital of C atom. C-C bond forms by overlap of two hybrid orbitals (the direction is along the axis between the atoms in the plain of molecule). This is s-bond. Two non-hybrid p-atom orbitals are overlapped higher and lower than the plain of molecule and, thus, π-bond forms.
The difference of a singe bond from a double bond:
- distance between carbon atoms at a double bond is smaller than at a single bond (0,134 nm); the corner between hybrid atom orbitals is 120°;
- electronegativity of hybrid C atom is 2,69;
- rotation around the line connecting C atoms is difficult;
- double bond is more solid as electron density on bounding molecule orbitals between carbon atoms increases (thermal firmness of ethylene is higher than ethane);
- high reaction ability of π-bond which is explained by the higher mobility of electrons outside molecule plain;
- increased electron density in comparison to single bond, on the periphery in particular. It leads to the fact that double bond attracts positively charged ions or polar molecules by its positive pole.
C-H bonds in acetylene refer to s-bonds, formed by overlap between s-orbital of hydrogen and hybrid sp-orbital of carbon; the molecule has one carbon-carbon s-bond (formed by overlap between two hybrid orbitals of carbon) and two carbon-carbon π-bonds (the result of crossing of two mutually perpendicular pairs of non-hybrid p-orbitals (py and pz) of carbon atoms).
Features of triple bond:
- carbon atoms, connected by a triple bond, have electronegativity equal 2,75;
- the length of CºC-bond is 0,120 nm;
- valent corners in acetylene on the basis of this model are 180° and molecule has a linear configuration, which makes cis-trans-isomery impossible at triple bond;
- the bond is strongly polar as in sp-hybrid form carbon atom holds electrons stronger than in sp2 and sp3-hybrid forms; consequently, electron pair in CH-bond in the molecule of acetylene is closer to the carbon nucleus than in the case of ethylene, and H atom has more mobility and has weak acid features (in contrast to alkanes and alkynes).
1.5. Copulative systems.
There are two types of copulative systems (and junctions)
1. π, π-junction. Here electrons are de-located between two (or more) multiple bonds. For example, in de-location of electrons in the bensole molecule 6 carbon  atoms participate, in butadiene – 4 atoms: СН2=СН–СН=СН2 «СН2–¾СН–¾СН–¾СН2
atoms participate, in butadiene – 4 atoms: СН2=СН–СН=СН2 «СН2–¾СН–¾СН–¾СН2

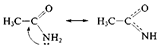




 2. p, π-junction. Here electrons of π-bond and p-orbital of hetero-atom take place in de-location: for example, acetamide and pirrole molecules:
2. p, π-junction. Here electrons of π-bond and p-orbital of hetero-atom take place in de-location: for example, acetamide and pirrole molecules:
In the case of acetamide molecule three atoms take place in de-location (C, O, N); in the case of pirrole molecule five atoms participate (four C atoms and one N atom). The longer the copulative system the more is its solidity. Aromatic system is a solid plain cyclic polien structure, containing (4n+2) π-electrons (n=1, 2, 3, …). For example, bensole is aromatic structure (6 π-electrons) and 1,3,5,7-cyclooktatetraen is not (8 π-electrons). Electron de-location is one of the important factors to increase the stability of molecules and ions, thus, this phenomenon is widely spread in biologically important molecules (vitamins, hem, chlorophyll, hemoglobin and others).
Types of carbon atoms. Atoms, connected to one carbon atom are called initial, correspondingly, three hydrogen atoms at initial carbon atom are also called initial. Carbon atom, connected to two carbon atoms is called secondary, and two hydrogen atoms bonded to it are also called secondary hydrogen atoms. Atom, connected to three other carbon atoms, is called tertiary as well as the only hydrogen atom bonded to it. Carbon atom connected to four carbon atoms is called quarternary.
1.6. Electron effects (inductive and mesomeric).
Molecule polarization is determined by different atom influence, which come in to the content of molecule. Atoms with high electronegativity attract electrons of s-bonds. Such type of polarization (displacement of electron density in s-bond under the influence the diversity in electronegativity of bonded atoms) is called inductive effect (it is marked by letter I and direct arrow ®). If in C-X bond electron density is displaced closer to the substitute X in relation to C-H-bond, then such substitute influence is called negative inductive effect (-I). If displacement goes closer to C atom in relation to C-H bond then such substitute influence is called positive inductive effect (+I). On transmission along the chain the effect goes out.

 Н Н
Н Н


 Н Сd+2 Сd+1 Сld- d+2 < d+1
Н Сd+2 Сd+1 Сld- d+2 < d+1
|
|
|
|
|
Дата добавления: 2014-01-11; Просмотров: 934; Нарушение авторских прав?; Мы поможем в написании вашей работы!