
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
The appropriate regulation of androgen activity is necessary for a range of developmental and physiological processes, particularly male sexual development and maturation
|
|
|
|
The nongenomic, rapid stimulation of second messenger cascades by androgens may ultimately exert biological effects through modulation of the transcriptional activity of AR or other transcription factors. Such modulation may occur through direct phosphorylation of transcriptional activators or their coregulators. The AR can also be activated in the absence of its cognate ligand, androgen by signaling pathways initiated by various growth factors.
In addition to the transcriptional or genomic mode of action by steroids, androgens, can also exert rapid, nongenomic effects. Nongenomic steroid activity typically involves the rapid induction of conventional second messenger signal transduction cascades. Nongenomic action of androgens can occur through multiple receptors.
Transcriptional activation by AR ultimately requires the recruitment of RNA Pol II (RNA polymerase-II) to the promoter of target genes. RNA Pol II recruitment is mediated through the assembly of GTFs (General Transcription Factors) to form the preinitiation complex, the first step of which is the binding of TBP (TATA box-Binding Protein) near the transcriptional start site. TBP binding induces DNA bending, which leads to the interaction between GTFs and steroid receptor-coregulator complexes. AR can also interact with a number of transcription factors including Activator Protein-1, SMAD3 (Sma and Mad Related Family), NF-KappaB (Nuclear Factor-KappaB), SRY (Sex-determining Region-Y).
A number of coregulators themselves perform enzymatic activities such as phosphorylation or acetylation, modifying either the chromatin surrounding the promoter of the target gene or other coregulators.
The transcriptional activity of AR is greatly modulated by coregulatory proteins. Coactivators such as ARA70 (Androgen Receptor Coactivator-70) and ARA55 stabilize the process of ligand binding to AR. The ability of AR to be translocated to the nucleus is regulated by several coregulators, for example, the F-Actin binding protein (Filamin). Inside the nucleus, AR interacts with DNA by targeting specific nucleotide palindromic sequences termed ARE (Androgen Response Element).
The unbound AR forms a complex with HSPs (Heat-Shock Proteins). The binding of androgens to AR induces dissociation of the AR from the HSPs and subsequent receptor dimerization and translocation into the nucleus, facilitating the ability of AR to bind to its cognate response element, and recruit coregulators to promote the expression of target genes.
Androgens mediate a wide range of developmental and physiological responses and are especially important in male sexual differentiation and pubertal sexual maturation, the maintenance of spermatogenesis, and male gonadotropin regulation. The principle steroidal androgens, testosterone and its metabolite DHT (5-Alpha-Dihydrotestosterone), mediate their biological effects predominantly through binding to the AR (Androgen Receptor), an androgen-inducible member of the nuclear receptor superfamily of transcription factors.
Androgen Receptor
A dramatic demonstration of the importance of estrogens in the regulation of fat deposition comes from transgenic mice that were genetically engineered to lack a functional aromatase gene. These mice have very low levels of estrogen and are obese. Obesity was also observed in estrogen deficient female mice lacking the follicle-stimulating hormone receptor. The effect of low estrogen on increased obesity has been linked to estrogen receptor alpha.
Structurally, AR can be subdivided into four functional domains: the NH2-terminal transactivation domain (or A/B domain), the DBD (DNA-Binding Domain), hinge region, and the LBD (Ligand-Binding Domain). An NH2-terminal AF1 (Activation Function-1), functions in a ligand-independent manner when artificially separated from the LBD, creating a constitutively active receptor. A ligand-dependent AF2 function is located in the LBD, which is responsible for an optimum transcriptional activation in response to the ligand.
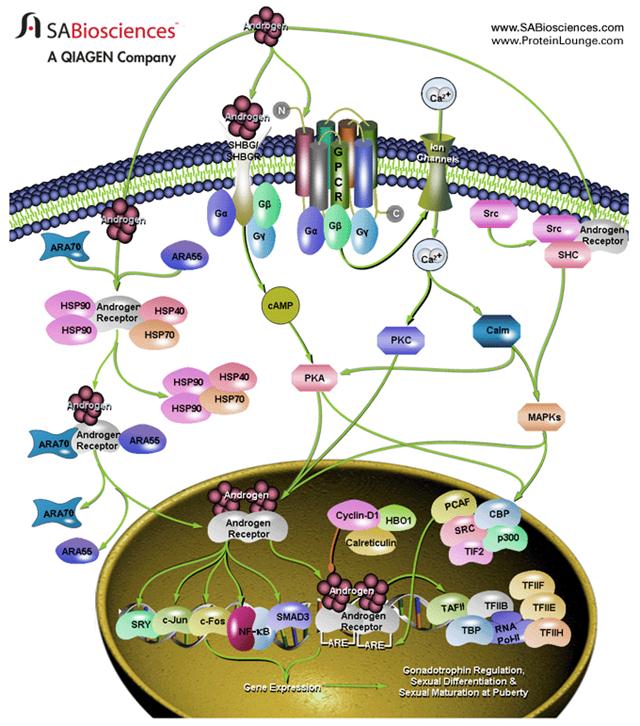
Among the coregulators, PIAS [Protein Inhibitor of Activated Signal Transducer and Activator of Transcription (STAT)] family of proteins and ANPK (Androgen Receptor-Interacting Nuclear Kinase) interact with and coactivate AR.
Transcriptional corepression of androgen-bound AR can be attributed to three corepressors: cyclin-D1, calcium-binding protein calreticulin and HBO1. The AR corepressor HBO1 is a member of the MYST protein family that is characterized by a homologous zinc finger and carries an acetyltransferase domain. Although AR is normally thought to function as a homodimer, it has been found to heterodimerize with other nuclear receptors including the ER (Estrogen Receptor), GR (Glucocorticoid Receptor) and TR4 (Testicular Orphan Receptor-4) and in each case result in a decrease in AR transcriptional activity.
Androgens can activate cAMP and PKA through the SHBG (Sex Hormone Binding Globulin)/SHBGR complex.
Androgens also stimulate an elevation in intracellular Ca2+ through a GPCR by activating an influx through nonvoltage-gated Ca2+ channels. The elevation of intracellular calcium activates signal transduction cascades, including PKA (Protein Kinase-A), PKC (Protein Kinase-C), and MAPKs (Mitogen-Activated Protein Kinase), that can modulate the activity of the ARs and other transcription factors.
AR also interacts with the intracellular tyrosine kinase c-Src, triggering c-Src activation. One of the targets of c-Src is the adapter protein SHC (SH2 Containing Protein), an upstream regulator of the MAPK pathway. The activity of AR and AR coactivators are influenced by direct phosphorylation by MAPK. AR phosphorylation by ERK2 is associated with enhanced AR transcriptional activity and an increased ability to recruit the coactivator ARA70. The SRC family of transcriptional coactivators: SRC1, SRC3, and TIF2 (Transcription Intermediary Factor-2) are targets of MAPK phosphorylation that results in an increased ability of these coactivators to recruit additional coactivator complexes to the DNA-bound receptor.
|
|
|
|
|
Дата добавления: 2014-01-14; Просмотров: 508; Нарушение авторских прав?; Мы поможем в написании вашей работы!