
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Laboratory Preparation of Hydrochloric Acid
|
|
|
|
Laboratory Preparation of Nitric Acid
Laboratory Preparation of Hydrogen
Read the commentaries to the lab experiment and then comment the formulas below.
The most convenient laboratory method for the preparation of hydrogen is based on the reaction of zinc with dilute sulphuric acid. The gas is to be collected over water. Zinc’s rate of reaction can be easily controlled by regulating the rate with which sulphuric acid is added.
 Zn+H2SO4 H2+ZnSO4
Zn+H2SO4 H2+ZnSO4
The surface of pure zinc placed in a solution of dilute sulphuric acid becomes coated with a film of hydrogen, the reaction proceeds very slowly. The evolution of gaseous hydrogen cannot be observed.
Nitric acid may be prepared by the reaction of concentrated sulphuric acid with sodium nitrate. In the laboratory method, a mixture of sodium nitrate and concentrated sulphuric acid is heated in a glass retort. Nitric acid is boiled out of the mixture and is condensed:
NaNO3+H2SO4 = HNO3+NaHSO4
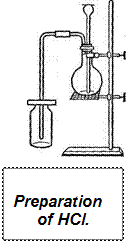
| Many experiments can be carried out in the laboratory of inorganic chemistry. Thus, if we want to obtain hydrogen chloride (HC1), which is often referred to as a hydrochloric acid gas, it is necessary to pour some sulphuric acid through a tube over the crystals of sodium chloride in flask. The flask is to be heated. On warming the flask, the hydrogen chloride is expelled as colourless gas with a suffoсating odour. |
It produces heavy clouds white fumes when it comes in contact with the moist air of the room. It is soluble and it cannot be collected over water as are oxygen and hydrogen. It is much heavier than the air and may be passed through a glass tube to the bottom of a bottle.

 Ca(OH)2 + 2NH4Cl = CaCl2 + 2NH3 +2H2O
Ca(OH)2 + 2NH4Cl = CaCl2 + 2NH3 +2H2O

 CH3CH2OH H2SO4Br2 CH2 = CH2 () + H2O
CH3CH2OH H2SO4Br2 CH2 = CH2 () + H2O
 Cu (OH)2 = CuO () + H2O
Cu (OH)2 = CuO () + H2O
4P + 5O2 = 2P2O5
17. Answer the questions:
1. What is the laboratory method for the preparation of hydrogen based on?
2.  Where is gas to be collected?
Where is gas to be collected?
3. How can the zinc's rate of reaction be controlled?
4. Can the evolution of gaseous hydrogen be observed?
|
|
|
|
|
Дата добавления: 2014-11-07; Просмотров: 1055; Нарушение авторских прав?; Мы поможем в написании вашей работы!