
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
How to read chemical formulas
|
|
|
|
SYMBOLS, FORMULAS AND EQUATIONS
Read the text and learn how to read chemical formulas.
Each of the 107 presently known chemical elements has a symbol. This symbol is usually derived from the name of the element. The symbol of oxygen is O, of hydrogen is H, of helium is He, of copper is Cu, of sodium is Na, of plutonium is Pu. Groups of symbols are called formulas. They are used to designate compounds. The formulae for water is H2O, for carbon dioxide CO2, for sulphuric acid H2SO4. These symbols and formulas are used to indicate chemical reactions. For example:
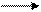 2H2O 2H2+O2
2H2O 2H2+O2
(Statement: Water decomposes to form hydrogen and oxygen)
The symbols of chemical elements are read according to the names of English alphabet letters.
The sign “+” is read as plus, and, together, with, react with.
The sign “-“ indicates one relation and isn't read at all.
The sign “=” is read as give, form or produce.
 The sign “ “ is read as give, pass over to or lead to.
The sign “ “ is read as give, pass over to or lead to.

 The sign “ ” is read as forms and is formed from.
The sign “ ” is read as forms and is formed from.
2H2O – a figure before an element signifies the number of molecules; two molecules of water.
 2H2+O2 2H2O
2H2+O2 2H2O
Two molecules of H two plus O two give two molecules of H two O or Two two-atom molecules of hydrogen react with one two-atom molecule of oxygen and produce two molecules of water.
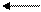
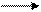 N2+3H2 2NH3
N2+3H2 2NH3
N two plus three molecules of H two form and are formed from two molecules of NH three or one two-atom molecule of nitrogen plus three two-atom molecules of hydrogen form and are formed from two molecules of ammonia.
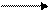 Na2CO3+CaSO4 Na2SO4+CaCO3
Na2CO3+CaSO4 Na2SO4+CaCO3
Na two Co three plus CaSO four form Na two SO four plus CaCO three or the sodium (Na) and the calcium (Ca) switch places. The sodium combines with the sulphate radical (SO4), forming sodium sulphate (Na2SO4) which dissolves in water. The calcium combines with the carbonate radical (CO3), forming calcium carbonate (CaCO3). Calcium carbonate does not dissolve in water and so settles to the bottom of the solution.
|
|
|
|
|
Дата добавления: 2014-11-07; Просмотров: 1660; Нарушение авторских прав?; Мы поможем в написании вашей работы!