
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Unit fuel cell
|
|
|
|
Introduction
A fuel cell is an electrochemical energy conversion device that converts hydrogen and oxygen into electricity, heat, and water. It is very much like a battery that can produce electricity while being recharged continuously. [1]
Although today's high-tech fuel cells hardly resemble their forefathers, the process upon which fuel cells are based has been known to science for more than 100 years. The first fuel cell (or "gas battery" as he called it), was invented by William Robert Grove in 1839, only 39 years after Alessandro Volto invented the battery (the voltaic cell). [1]
Fuel cell converts chemical energy in fuels into electrical energy directly, promising power generation with high efficiency and low environmental impact. Because the intermediate steps of producing heat and mechanical work typical of most conventional power generation methods are avoided, fuel cells are not limited by thermodynamic limitations of heat engines such as the Carnot efficiency. In addition, because combustion is avoided, fuel cells produce power with minimal pollutant. [2] These criteria are very attractive for automotive industry.
2. Fuel cell’s operation principles
Unit cells form the core of a fuel cell.. The basic physical structure, or building block, of a fuel cell consists of an electrolyte layer in contact with an anode and a cathode on either side. Figure 1 describes this.
Schematic representation of a unit cell with the reactant/product gases and the ion conduction flow directions through the cell is shown in the figure 2, below.
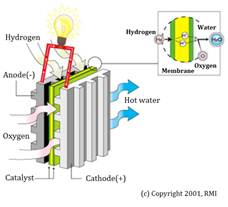
Fig. 1 - Basic structure of a unit fuel cell
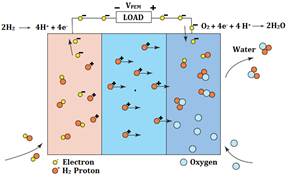
Fig. 2 – Description of internal processes
In a typical fuel cell, fuel is fed continuously to the anode (negative electrode) and an oxidant (often oxygen from air) is fed continuously to the cathode (positive electrode). The electrochemical reactions take place at the electrodes to produce an electric current through the electrolyte, while driving a complementary electric current that performs work on the load.
|
|
|
|
|
Дата добавления: 2014-12-24; Просмотров: 395; Нарушение авторских прав?; Мы поможем в написании вашей работы!