
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Scan the biography. Put questions to the given answers
|
|
|
|
Robert Boyle was born January 25, 1627, in Ireland and died December 30, 1691, in London. He was a chemist and a natural philosopher. He was noted for his pioneer experiments on the properties of gasses. (Turn to page 107 for further information). Sent to Eton College in 1635, Boyle read Galileo's works during a European tour (1639-44). At Oxford he took part in constructing an air pump. Beginning in 1668, he lived in London and continued experimental work on the calcination of metals and the distinction between acids and alkalis. Celebrated during his lifetime, Boyle's law called Mariotte's Law in Europe (by the name of French physicist (who also recognized a relationship of volume and pressure), is the relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by Boyle in 1662, states that the pressure (p) of a gas varies inversely as its volume (v) at constant temperature; in equation form, pv=k, and is a constant. The law can be derived from the kinetic theory of gasses assuming a perfect (ideal) gas. Real gasses obey Boyle's law at sufficiently low pressures, although the product pv generally decreases slightly as the gas pressure is raised to appreciably higher values.
1.-------------------------- No, he was both a chemist and a philosopher.
2.----------------------- On the properties of gases.
3.------------------- Yes, he was celebrated during his lifetime.
4.--------------- Boyle’s Law and Mariotte’s Law mean the same.
5.----------- They were Irish and French.
6.-------- From the kinetic theory of gases.
7.----- Real gases.
8.-- At sufficiently low pressures.
21. Various pumps are widely used in vacuum industry. Each pump has its name and symbol according to the standards. Study some of the following pump signs in Russian. Be ready to read about them in English.
| I. Обозначения вакуумных насосов (ГОСТ 2. 796 – 95) | |
| 1.1 Насос вакуумный. Общее обозначение | 
|
| 1.2 Насос вакуумный механический. Общее обозначение | 
|
| 1.2.2 Турбомолекулярный | 
|
| 1.2.4 Водокольцевой | 
|
| 1.4.1 Насосы вакуумные адсорбционные. | 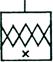
|
| 1.4.4 Криогенный | 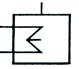
|
| 1.4.5 Испарительно-ионный | 
|
| 1.4.6 Магнитный электро-разрядный | 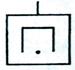
|
| 1.4.7 Комбинированный | 
|
Follow-up Activities
22. Look through the texts of Unit IV again and make notes under the following headings. Then use your notes to talk about it.
1. Magnetron History (additional facts).
2. Vacuum Tube History.
3. Magnetron Operation.
4. Magnetron Applications.
5. A Vacuum Technology Scientist (Robert Boyle).
Unit V. Vacuum Pumps:
Operation, Classification & Application
Before you start: Think it over. Agree-Disagree
“There is no subject so old that something new cannot be said about it.” –
Dostoevsky Fyodor Mikhailovich
Note: Fyodor Dostoevsky, 1821-1881, Russian novelist
Reading, Vocabulary & Creative Practice
|
|
|
|
|
Дата добавления: 2014-12-26; Просмотров: 506; Нарушение авторских прав?; Мы поможем в написании вашей работы!