
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Methodical guidance for laboratory work №1
|
|
|
|
Methodical recommendation for the laboratory lesson
SEMEY STATE MEDICAL ACADEMY
Specialty: 0501301General medicine
Discipline: chemistry
Chair: of chemistry and technology of medicine
Higher base medical education, course – 1
Topic №13: Carbohydrates. Monosaccarides.
Semey 2007
Approved
on the chair meeting from “_____”____________2007.
Protocol № ____
Head of the chair of chemistry and technology of medicine,
prof. __________ Gavrilenko I.V
1.Topic№13: Carbohydrates. Monosaccharide.
2.Aim: to studyof classification, structure of isomerism and chemical properties of monosaccharide.
3.Objectives of teaching: to teach it is correct to write carbonyl formula of Fisher, half-acetylic formula of Colly-Tollence and cyclic formula of Cheourse the most important monosaccharide, fulfil the qualitative reaction of monosaccharide and write the equations of reactions. Study of topic to form of student of the knowledge stereo- chemical stucture, tautomer forms and the most important properties of monosaccharide as the base for metabolic processes understanding.
4. Basic questions:
1.Carbohydrates.Concept. Classification.
2. Structure of monosaccharide, classification.
a) carbonyl formula of Fisher
b) half-acetylic formula of Colly-Tollence
c) cyclic formula of Cheourse
3. Isomerism of monosaccharide (stereoisomerism, cyclo-, oxo-tautomerism, conformation).
4. Chemical properties of monosaccharide.
а) oxidation of monosaccharide (gluconic, glucaric, glucoronic acids)
b) reduction of monosaccharide
c) formation of О- and N-glycosides
d) formation of ether
5. Qualitative reactions on monosaccharide
6. Derivatives of monosaccharide: deoxysacarose, aminosacarose, neiramine acid, ascorbic acid.
5. Methods of teaching:
The determining initial level knowledge’s of student on chemistry. The conversation and questioning on topic of the lesson. Fulfilling the laboratory work and protection of the report.
Experiment №1 Trommer's reaction
Fill t-t with 5-6 drops 1% solution of glucose and add 2-3 drops 10% solution of NaOH and add 5% solution of copper sulfate till formation of muddiness. Heat the t-t and watch the formation of red or yellow p.p.t. In the Trommer's reaction the oxidizing agent is copper hydroxideCu(OH)2 which during the reaction distracts into copper hydroxide (I) and then into copperhydroxide(I) 1 and then into copper oxide (I) red p.p.t. Write the equation of the reaction.
СuSO4 + 2NaOH ® Cu(OH)2↓ + 2Na2SO4
Blue p.p.t.
С6Н12О6 + 2 Cu(OH)2 → product of oxidation + 2CuOH + Н2О
yellow p.p.t
2CuOH → Cu2O + Н2О
red p.p.t
Make conclusion.
Experiment №2. Pheling's reaction.
Pheling's reaction is a mutating reaction of Trommer and differs from it that for oxidation of the glucose use the reagent Pheling's, in composition which enter the copper sulfate (II) CuSO4, segnet salt and NaOH. In this reagent copper is found in the manner of compounds with segnet salt, due to that hydrate of copper oxide does not precipitate.
Pour in the t-t 5-6 drops 1% solution of glucose and add 3-5 drops of Pheling's reagent and heat. There is red or yellow p.p.t.
Reaction is similarly Trommer's reaction. Write the equations of reactions. Indicate, what properties shows the glucose in this reactions. Make a conclusion.
Experiment 3. Selivanofs reaction of fructose.
Pour in the t-t 5-6 drops of fructose and 2-3 drops of Selivanofs reagent(of resorcinol and 100ml of hydrochloric acid). Boil the mixture for 2-3 minand watchthe formation of red color. Ketohexoses by heating with hydrochloric acid lose water molecules with formation of oxymethylfurfurol which gives with resorcinolproducts of condensation of red color.
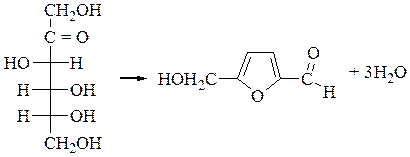
fructose oxymethylfurfurol
Make a conclusion.
Experiment №4. Reaction of penthouses.
Tollense reaction: pour in the t-t sawdust where polysaccharides present andmoist them with
 |
concentrated hydrochloric acid and add some crystals of fluoroglucine. There is red color. Pentosanes presenting in sawdust are destroyed till penhoses by action of hydrochloric acid. Penthouses by action of hydrochloric acid lose the water and furfurol is formed. The reaction is:
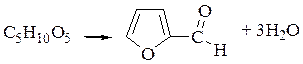
6.Literatura:
1. J. M. Sehgal. Modern Chemistry, class XI, Delhi, 1989 - 15 items.
2. Jean B. Umland & John M. Bellama, General Chemistry. Houston. USA. 1996 - 1 item.
3. Darrel D. Ebbing, General Chemistry, Wayne University, USA. 1990 - 3 items.
4. Karen C. Timberlake, Chemistry - An Introduction to General. Organic and Biochemistry, Los Angeles, USA, 1991-1 item.
7. Control:
1. What products are formed at interaction α-D-glucopiranose with methanol in acid medium? Write the scheme of reactions by formula of Cheourse.
2. What products are formed at interaction α-D-galactopiranose with ethanol in present acid catalysis? Write the scheme of reactions by formula of Cheourse.
3. Write the reaction of the reception ethyl α-D-mannopironose. Possible as source monosaccharide use β-D-ribofuranose?
4. Write the hydrolysis reaction ethyl-α-D-glucopiranose in acidic medium.
5. Write the hydrolysis reaction methyl-α-D-galactopiranose using formula of Cheourse.
6. Write the reaction of the reception of glycoside at interaction methanol with α-D-glucopiranose, α-D-galactopiranose. Write the hydrolysis reaction of the got glycoside. Why necessary acid catalysis?
7. What properties of glucose shows in reactions "silver mirror"? On than is founded determining of glucose in biological liquid?
8. Indicate, what substance are formed when reduction of monosaccharide (glucose, mannose, xilose, galactose) by hydrogen on nickel or palladium. Name the product of reduction.
|
|
|
Дата добавления: 2015-07-13; Просмотров: 904; Нарушение авторских прав?; Мы поможем в написании вашей работы!