
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Monitoring water pollution
|
|
|
|
WATER POLLUTION AND PROTECTION
Economics in relation to the water resource can be either consumer or user.
Consumer: takes water from a source, uses it for needs of production and returns it but in the other place, less quantity and different quality.
User: doesn’t take water from a source, but uses it as media (shipping, fishing, sport) or source of energy (hydro-power stations). However they change water quality also.
Water pollution is classified as:
Chemical pollution: is caused by harmful non-organic (acids, alkaline, mineral saline) or organic (oil, oil-products, surface active substances, washers, pesticides) substances incoming to water surface layers. Most of non-organic substances are toxic for community inhabiting waters (compounds of arsenic, lead, mercury, copper, cadmium, chromium, fluorine). They are absorbed by plankton and then transmitted through feed links to other organisms. It’s accompanied by cumulative effect that 10 times increases amount of harmful compounds in every next feed link. Metal manufacture, mineral resource industry, chemical industry, agriculture (fertilizers) are the main sources of mineral pollution. Sewages of chemical plants contain great quantity of organic compounds. Most of synthetic washers contain phosphorus. Increased quantity of phosphates in sewages causes intensive growing of blue-green water plants - water “blossom” what lowers oxygen in water and kills water animals.
Physical pollution: changes physical characteristics of water – clarity, contents of suspension and insoluble agents, radioactivity. Power stations emitting thermal waters are sources of thermal pollution (especially Nuclear Power Stations, t = 45 deg C).
Biological pollution: is caused by microorganisms incoming to water through sewages (virus, bacteria, fungus, protist). Biological pollution is examined by the following parameters:
1. “koli-index”: quantity of intestinal bacillus in 1 Liter of water (TLV = 3);
2. biochemical oxygen consumption (BOC): amount of oxygen needed to decompose organic substances into inorganic (BOC TLV = 3 mg/l in potable water within 5 days).
Water protection:
1. recycling;
2. wasteless technologies;
3. burying sewages;
4. purifying water;
5. reducing use of chemicals in agriculture;
6. imroving tankers.
To provide normal life activity of the human’s organism it’s important to know the concentration of harmful substance in solutions (for example in sewage). This is the photoelectric method of quantitative analysis based on capability of the investigated substance to absorb the electromagnetic waves in optical range applied for.
Colorimetry is one of the photometric methods of analysis. The essence of the method consists in coloring a component (if it was colorless) and determining its concentration by the quantity of light it absorbs.
The main law of colorimetry - Bouger-Lambert-Behr law states dependence between luminous flux a solution absorbs and concentration of a substance which absorbs the light in it:
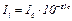 , (3)
, (3)
where 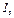 ,
,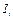 - intensities of the luminous flux falling on solution and passed through the solution with thickness
- intensities of the luminous flux falling on solution and passed through the solution with thickness 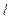 correspondingly,
correspondingly,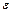 - molar coefficient of light-absorbing, or extinction, which doesn’t depend on concentration of investigated substance, but depends on its nature, wavelength of the luminous flux and temperature, C - concentration of the substance absorbing the light, gram-molecule/liter.
- molar coefficient of light-absorbing, or extinction, which doesn’t depend on concentration of investigated substance, but depends on its nature, wavelength of the luminous flux and temperature, C - concentration of the substance absorbing the light, gram-molecule/liter.
Optical density of solution is calculated by the formula:
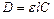 . (4)
. (4)
What means that if the light-absorbing obeys to Bouger-Lambert-Behr law, then optical density of solution is directly proportional to concentration of substance in it. In that case the optical density D dependence on concentration C is linear beginning form coordinate origin, as shown in the fig 2.
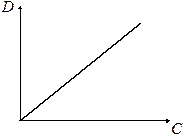
Fig. 2 Optical density dependence on concentration by Bouger-Lambert-Behr law
Various substances absorb light waves with various wavelength differently. If to draw a graph of dependence of substance’s optical density D on wavelength l of a light going through a substance, that graph will be a curve to have maximum and minimum. The measuring of optical density should be carried out on such a wavelength which corresponds to the maximum light absorbing in investigated substance. Here is the highest sensitivity and fidelity of measuring achieved. Needed wavelength is picked out by means of light filter which is selected so that it passes through the light of that part of the spectrum where investigated substance absorbs light maximally. In other words, minimum of the light filter absorbing is to coincide with maximum absorbing of investigated substance, as it’s shown in fig. 3.

Fig. 3 For measuring optical density of substance by means of light filter:
1 - light filter absorbing, 2 - substance absorbing.
Optical density if additive value, i.e. consists from optical densities of all the components present in solution:
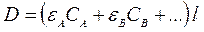 . (5)
. (5)
If for comparison to place in the cell solution containing all the components in the same concentrations that investigated solution has but without component which is to be found then optical density of that component will be defined. That’s why solution placed in cell for comparison is called zero-solution. In case of two component system the zero-solution is a solvent, for example water.
There’re couple of ways to analyze light absorbing. Quite simple and convenient, especially for serial measuring, is method of calibration curve. The essence of method consists in the following. First the optical densities of a number (5-10) of solutions with known concentration (here’re mentioned solutions of the substance concentration of which needs to be found) are measured. Then calibration graph is built tracing on ordinate axis optical density, and on abscissa axis - concentration, as in fig. 2.
When it’s done optical density of investigated solution will measured, and its concentration will be found by the graph. If in some range of concentration calibrated graph gets deflected from linear, i.e. the deviation from Bouger-Lambert-Behr law, then measuring are carried out in concentration, where that law is fulfilled.
The reasons why Bouger-Lambert-Behr law is not fulfilled can be various. In some cases dependence D(C) deflects from the line when not monochromatic light is used. Dissociation, polymerization of colored components, their interaction with solvent or other components of solution also influence on light absorbing. Frequently solution’s color and optical density depend on pH-value. Optical density changes can occur in consequence of coagulation, in some cases because of component destruction under the light influence etc. That’s why only newly prepared solutions are used or they are added by stabilizers. If measuring is distorted by outside colored substances, measuring is carried out on such wavelength in which those substances don’t absorb light, or they are masked.
For determining concentration of harmful substances in solutions concentration photoelectric colorimeter «КФК-2» can be applied.
It’s implemented to measure, in separate parts of wavelength diapason 315.. 970 nanometer which is picked out by light filters, light-transmission factor and optical density of liquid solution and solid bodies, and also determining concentration of substances in solutions by drawing calibration graphs.
Colorimeter «КФК-2» allows to measure light transmission factors of suspension dispersion, emulsion and colloidal solution in light passing through. It’s applied at water-supply factories, in metallurgy, chemical, food industry, agriculture, medicine etc.
Electrical scheme consist from light-electrical signal converters (photodetector), measuring amplifier of direct current, voltage stabilizer 63 V (supplying illuminating lamp) and 62 V (supplying photoelectric cell), and also power supply ±18 V (supplying measuring amplifier of direct current).
Photodetectors and direct current amplifiers with all control and connecting components are set in optical block, and voltage stabilizers with supplying transformer - in power unit.
Optical block contains: illuminant; frame with optical instruments; light-filters; cell holder; photometric device with direct current amplifier and control buttons; indicator.
The general view of colorimeter is shown in fig. 4.
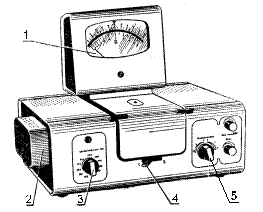
Fig. 4 Colorimeter «КФК-2»:
1 - microammeter; 2 - illuminant; 3 - light-filters switch; 4 - cell switch in luminous flux; 5 - sensitivity switch (amplification factor).
Light-filter is moved into the luminous flux by knob 3.
Cells are switched in luminous flux turning knob 4 against stop, and photodetectors - knob 5.
Microammeter 1 graduated in light-transmission factor T and optical density D scale is as the indicator implement.
Switching light-filters knob 5 «Чувствительность» should be turned into minimum position (minimum sensitivity). That prevents indicator from overload and breakage.
Measuring with light-filters 315, 364, 400, 440, 490, 540 nanometer, marked on bezel in black color, knob «Чувствительность» is set into one of the positions «1», «2», «3» marked on bezel the same color.
Measuring with light-filters 590, 670, 750, 870, 980 nanometer, marked on bezel in red color, knob «Чувствительность» is set into one of the positions «1», «2», «3» marked on bezel the same color.
Do not touch with your fingers working section of cell surface (lower liquid level in cell) placing cells into cell holder.
Contamination and solution drops on working section bring about obtaining error data. Liquid should be poured into the cell up to the mark on the side wall of cell. Sometimes liquid in the limited space of cell forms meniscus. It rises to a significant height equal to 4.. 6 mm through capillaries and especially through cell edges. If a liquid level exceeds the mark on the side wall of cell, creeping over the edges are observed, what makes an image of cell leakage.
Do not cline the cell with liquid placing it into cell holder.
After light-filter was switched or when the cell block was uncovered for some time (over 5 minutes) measuring can be started passing 5 minutes of photodetector light-striking.
Finishing colorimeter operation before power is off knob «Чувствительность» should be placed in position 1, marked in red color, and knob «Установка 100 грубо» - in ultimate left position, only when it’s done turn the power off (switch «Сеть»).
Preparing to work includes steps listed below.
1.Take into volumetric flask (beginning from volumetric flask #1) accordingly 2; 4; 6; 8; 10 ml of standard solution of colored component. Then add distilled water into each flask (up to lower mark on flask neck) and mix water with the component. That way each flask contains solution of colored component with different concentration. Pour the researched solution into the volumetric flask.
2.Turn on colorimeter for 15 minutes before starting measurement. During warm up period the cell block should be opened (shutter over the photodetector shields luminous flux).
3.Pick appropriate to measuring color light-filter out (670 - red color).
4.Set minimum sensitivity of colorimeter. This is to turn the knob «Чувствительность» into position «1» (red color), knob «Установка 100 грубо» - into ultimate left position.
5.Before every measuring and switching photodetectors check the colorimeter indicates «0» in the scale of light-transmission T when the cell block is opened. When indication is shifted from the «0» position it’s to be adjusted by grooved potentiometer.
Measuring optical density:
1. place into luminous flux cell with a solvent (water), relative to which the measuring is carried out;
2. cover the cell block;
3. set «0» in colorimeter scale by knobs «Чувствительность», «Установка 100 грубо» and «Точно». The knob «Чувствительность» can take any of three positions «1», «2», «3»;
4. replace the cell with solvent or control solution by the cell with researched solution turning the knob 4;
5. record indication of D scale for 5 standard and researched solutions;
6. carry on measuring for 3-5 times and take the mean value of all obtained data as the result.
Steps to determine concentration of a substance are:
1. pick the light-filter out;
2. pick the cells out;
3. build the calibration graph for given substance;
4. measure the optical density of researched solution and determine concentration of substance in given solution.
Calibration graph is drawn as following. Prepare the range of solutions with known concentrations, covering range of possible concentrations of this substance in researched solution.
Measure optical densities of all the solutions and build a calibration graph, tracing on abscissa axis known concentrations and on ordinate axis - corresponding to them optical densities.
Further built calibration graph is used to determine unknown concentration of the substance in researched solution. For it pour the solution into the same cell, calibration graph is built for, and switching the same light-filter determine optical density of solution. After it find the concentration corresponding to measured value of optical density in calibration graph.
Practice:
Problem 1
Find concentration of X component in solution using following data: optical density of X component in researched solution = 2.5; optical densities of calibration solutions: D1= 4; D2 = 6; optical density of solvent = 2; concentrations of X component in calibration solutions: C1= 10 mg/l; C2 = 35 mg/l.
Problem 2
Find optical density of X component in solution using following data: concentration of X component in researched solution = 30 mg/l; optical densities of calibration solutions: D1 = 3; D2 = 4; optical density of solvent = 1; concentrations of X component in calibration solutions: C1 = 10 mg/l; C2 = 45 mg/l.
|
|
|
|
|
Дата добавления: 2014-01-04; Просмотров: 702; Нарушение авторских прав?; Мы поможем в написании вашей работы!