
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Methods for monitoring nitrate concentration
|
|
|
|
Monitoring food quality is carried out by:
- manufacturer;
- special official establishments - sanitary-epidemic stations;
- public organizations.
For detection of nitrates there is a whole arsenal of research tests:
We’ll focus in POTENTIOMETER method for nitrate contents determining applied in this work.
Let’s take two vessels and fill them with any solutions of potassium niter having different concentrations then connect them with thin pipe which have ion-selective membrane and capable to pass only ions 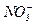 through (fig. 5).
through (fig. 5).
Dipping into solutions two electrodes connected to milli-voltmeter it’ll indicate an electromotive force (EMF). This is electrochemical circuit, which work as a battery.
If to leave concentration of a saline solution in the first vessel unchanged and to change concentration of solution only in second one, the magnitude of EMF will be changed accordingly. It is possible to build a calibration curve giving EMF dependence on concentration of saline solution in the second vessel.
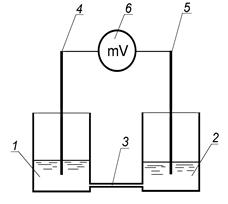
Fig. 5 The scheme for nitrates concentration determining by potentiometer method:
1,2 - vessels with saline solutions, 3 - pipe with ion-selective membrane, 4,5 - electrodes (metallic wire), 6 - milli-voltmeter.
That curve can be described by the equation
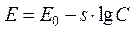 , (6)
, (6)
where E - magnitude of electromotive force, mV; E0 - constant, mV; s - constant coefficient (slope of a curve); C - saline concentration in the second vessel, mg/l.
Obtained graph can be further applied for determining concentration of potassium niter in solution (in the second vessel) with unknown concentration.
Solving equation (6) for C we’ll get the following equation for concentration determination:
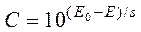 . (7)
. (7)
Instrument and equipment applied in laboratory analysis are shown in fig. 6.
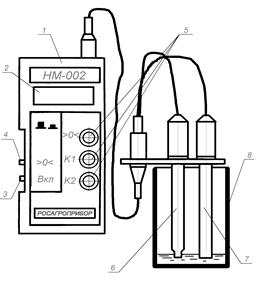
Fig. 6 Nitrate-meter «HM-002»:
1 - transducer; 2 - indicator; 3 - switch; 4 - mode switch; 5 - knobs of variable resistance; 6 - auxiliary electrode; 7 - measuring electrode; 8 - vessel with solution.
The method of potentiometry described above is the basic for the device operation.
The device is purposed for the express analysis of nitrates in water solutions of ground, water, agricultural products.
It consists from two parts: transducer and electrode system. The transducer 1 provides the output of concentration value on indicator 2, not the value of electromotive force what’s measured. That makes device more convenient in use.
Instrument should be calibrated before use. To do that use 3 solutions with known concentration of potassium niter KNO3: C1 = 10 mg/l, C1 = 1000 mg/l, C3 = 100 mg/l for device calibration. Dip the electrodes washed out in distil water and wiped by filter paper into solution with concentration of ions C1 = 10 mg/l. After 1.5.. 2 min press the button «> 0 <» and set on the device indicator «0.00 m».
|
|
|
|
|
Дата добавления: 2014-01-04; Просмотров: 478; Нарушение авторских прав?; Мы поможем в написании вашей работы!