
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Selenium, telurium, and polonium
|
|
|
|
Compounds of elements (-2)
Apart from the simple hydrogen chalcogenides of Se and Te (which are more acidic and thermally less stable than the corresponding sulfur analogs) the most stable inorganic compounds of selenium (and its higher homologs) are:
1. Chalcogenides and polychalcogenides with strongly electropositive ions such as alkali or alkaline-earth metals, and those that are more covalently bonded with transition metals;
2. Large variety of Se(II), Se(IV), and Se(VI) compounds with electronegative elements O, F, Cl, and Br;
3. Homologs belonging to the interesting class of sulfur–nitrogen compounds;
4. Organoselenium compounds.
When heated, selenium and tellurium react with metals forming selenides and tellurides:
2K + E° = K2E-2; 2Al + 3E = Al2E3-2
Thus active metals form salt-like selenides and stoichiometric tellurides, which dissolve in water readily.
Reactions of Se and Те with transitional metals give selenides and tellurides of various types МЕ, М2Е3, М3Е4, МЕ2 and etc., with many of them having a varying composition. At low content of non-metallic elements they have mainly metallic nature, at high content of chalcogens — semiconductors.
Hydrogen compounds. Along with H2S hydrogen selenide, H2Se, and hydrogen telluride, H2Тe, are known. Hydrogen polonide, H2Ро, is extraordinarily unstable. Radioactivity of a gas formed after HCl treatment of polonium coated magnesium can serve as a proof. Polonides (Na2Po) are also known.
Preparation. The action of diluted HCl or Н2О on selenides and tellurides is used in a laboratory:
Al2E3 +6HCl = 3H2E + 2AlCl3
Al2E3 +6H2O = 3H2E + 2Al(OH)3
Н2Se can be prepared if gaseous Н2 passes over heated to 400°C elemental selenium.
Properties. Under ordinary conditions H2Se and H2Тe are colourless toxic gases with a very unpleasant smell. Their structure and properties resemble those of H2S:
| H2О | H2S | H2Se | H2Te | |
| m.p., °С | -85.6 | -65.7 | -51 | |
| b.p., °С | -60.4 | -41.4 | -2 | |
| Bond length, nm | 0.096 | 0.133 | 0.146 | 0.169 |
| Bond energy, J/mol | ||||
| Valence angle H-E-H | 104.5° | 92.2° | 91.0° | 90° |
| DН°f,298, kJ/mol | -285.8 | -20.6 | 99.7 | |
| DG°f,298, kJ/mol | -237.2 | -33.8 | 19.7 | 85.2 |
| K1(aqueous solution) | 1.8•10-16 | 1•10-7 | 1.9•10-4 | 2.3•10-3 |
Covalent radii of chalkogens grow in the series H2О—H2S—H2Se—H2Tediminishing Н-Е bonds energy and H2Е molecules stability. That is why unlike H2О and H2S, selenium and tellurium compounds with hydrogen are endothermic compounds (DН°f>0), they readily decompose when heated: thermal decomposition of H2Se takes place with a noticeable rate at to > 300°, and H2Тe is gradually decomposed already under ordinary conditions.
Similarly to molecule H2S the molecule of H2Se and H2Te have bent shape with valence angles 91° and 90°, which signifies that hybridization of selenium and tellurium orbitals is absent.
Solubility of H2Se and H2Te is better as compared to H2S. In aqueous solutions they display properties of weak hydroselenic and hydrotelluric acids. The strength of these hydracids gradually grows in the series H2О-H2S-H2Se-H2Te. It is explained by diminishing of Н — Е bond energy and growth of their ability to polarization. Dissociation of compounds is hereupon facilitated under the action of polar molecules of water:
Н2Е 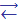 Н+ + НЕ-
Н+ + НЕ-
HЕ- 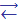 Н+ + Е2-
Н+ + Е2-
H2Se can form two types of salts: neutral and acidic. H2Te has no reactions when acidic salts are the products. Exposed to air oxygen their solutions quickly acquire a red color owing to the formation of polyselenides and polytellurides of metals, similar to polysulfides:
12K2Te + 5O2 + 10H2O = 2K2Te6 + 20KOH
Neverthless, as a result of large size and low electronegativity of elements, a reaction between selenides of different chemical nature, as well as between tellurides with the formation of analogues of thiosalts can not be realised.
In the series H2S-H2Se-H2Te the reducing activity grows appropriately. So, sulfur is able to oxidize H2Se аnd H2Te:
H2Se-2 + S° = Se° + H2S-2,
and oxygen oxidizes hydrogen compounds and their derivatives of other elements of the subgroup:
2H2Е + O2 = 2E + 2H2O (in solution)
2H2Е + 3O2 = 2EО2 + 2H2O (burning)
|
|
|
|
|
Дата добавления: 2014-01-11; Просмотров: 488; Нарушение авторских прав?; Мы поможем в написании вашей работы!