
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Thermal decomposition or reduction of gypsum СaSО4.2H2O
|
|
|
|
Oxidative combustion of sulfides of metals (pyrites)
Oxide of sulfur (IV). Preparation.
In industry:
4FeS2 + 11О2 = 2Fe2О3 + 8SО2
2. Incineration of native sulfur (in countries with rich deposits of elementary sulfur):
S + О2 = SО2; DН0298 = -296.8 kJ/mol
2СaSО4  2СаО + 2SО2 + О2
2СаО + 2SО2 + О2
СaSО4 + С  СаО + SО2 + СО2 (in cylinder furnaces)
СаО + SО2 + СО2 (in cylinder furnaces)
In laboratory: 1. Using sulfites:
Na2SО3 + 2H2SО4 = 2NaHSО4 + SО2 + H2O,
2. Reduction of concentrated H2SО4 by low active metals (e.g. with copper):
Cu + 2H2SО4(conc.) = CuSО4 + SО2 + 2H2O
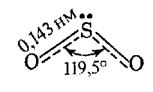
Structure. SО2 has a bent structure (S–O bond length 143.2 pm, O–S–O valence angle 119.5◦ in the gas phase),
|
that allows to ascribe for the sulfur atom sp2- hybridization (theoretical angle 120о). Bonds of the central sulfur atom with every oxygen atom have equal energy (497.5 kJ/mol) and length. According to the Valence Bond method, the atoms in SО2 are bound by two s-bonds and by a delocalized three-centered p-bond.
The state of hybridization of sulfur orbitals in SО2 corresponds to the structure draft:

The formation of s-bonds in SО2 molecule is a result of overlap of two sulfur monoelectronic hybrid orbitals with 2рх monoelectronic orbitals of two oxygen atoms. To achieve an octet electronic configuration 2рz -orbital of the oxygen atom accepts one electron of unhybridized 3рz -orbital of sulfur (electron transition is shown on the chart below with an arrow):
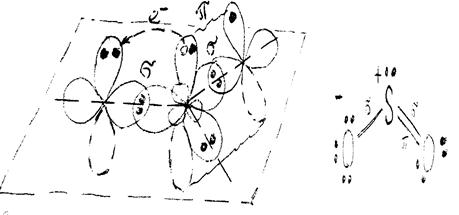
This arrangement results in the formation of a p-bond between sulfur and the second atom of oxygen. The resonance structure of this overlapping state of atomic orbitals is shown near the chart. However, this structure has no similarity to actual SО2 structure since S-О bonds are identical in the real molecule. The same is the description of validity of the opposite resonance structure of overlapping orbitals.
 |
According to the resonance theory a real molecule is an intermediate state between the limiting cases of resonance structures, and its structure is the result of imposition (superposition) of all variants of resonance structures. Therefore, the real structure of SО2 corresponds to the first structure chart, where together with s-bonds delocalised three-centred p-bond is present.
Properties. SO2 is a poisonous, colourless gas with a sharp smell; it condenses (−10 ◦C) to give a colourless liquid and ultimately (−76 ◦C) white crystals. SO2 is an acid oxide, its molecule is polar (m = 1.59 D = 0.53•10-29 C•m).
SO2 demonstrates reducing properties (that are more characteristic):
SO2 + Br2 + 2H2O = H2SO4 + 2HBr,
and oxidizing properties:
SO2 + 2H2S = 3S + 2H2O.
It is well soluble in water (36 volumes SO2 in one volume of water at 20 oC) with the formation of hydrates of variable composition SO2∙nH2O. Some dissolved molecules react with water and form sulfurous acid, Н2SO3.
SULFURUOUS ACID, H2SO3.
SO2 + H2O 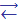 Н2SO3
Н2SO3
Н2SO3 exists only in diluted solutions as a result of reversibility of this reaction. Its equilibrium is significantly shifted to the left hand side. On being heated, the solubility of SO2 diminishes and the equilibrium is even more displaced toward reagents. A reaction of acids with salts of sulfurous acid causes evolving SO2 because of Н2SO3 instability; therefore, Н2SO3 cannot be isolated in the free state.
Structure of sulfite-ion, SO32-. The structure is pyramidal with the atom of sulfur on the top.
The value of valence angles (Ð 1050) is the evidence of sp3-hybridization. All bonds here are equivalent, and their length (151 pm) is somewhat longer of SO2 bonds (143 pm). At bonds formation, every atom surrounds electron octet that provides SO32- ion stability. The identity of S—O bonds suggests equivalent distribution of two negative charges between three atoms of oxygen.
Diminishment of valence angle in the sulfite ion (105o) in comparison with tetrahedral (109o28') is caused by the electrostatic repulsion between the unshared electron pair and the bond electrons.
Since the unshared electron pair of sulfur is located on spatially directed hybrid orbitals, the sulfite ion is an active donor of electron pair and its transformations into tetrahedral ions НSO3- and SO42- can be realised easily.
Properties. Sulfurous acid is a dibasic acid of intermediate strength:
Н2SO3  Н+ + НSO3-; K1 = 2∙10-2;
Н+ + НSO3-; K1 = 2∙10-2;
Н2SO3-  Н+ + НSO32-; K2 = 6∙10-8.
Н+ + НSO32-; K2 = 6∙10-8.
It forms two types of salts: sulfites and hydrogen sulfites:
NaOH + SO2 = Na2SO3
Na2SO3 + SO2(excess) + H2O = 2NaНSO3
The hydrogen sulfite-ion, НSO3-, has two tautomeric forms:
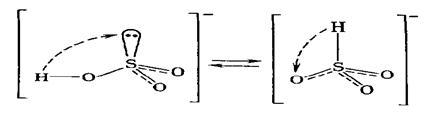
The first structure corresponds to generally neutral salts, the second structure is close to the salts of some low active metals.
Only sulfites of alkali metals and hydrogen sulfites are well soluble. Only solid sulfites can be prepared. Solid hydrogen sulfites are known for alkali metals only.
Salts of Н2SO3 are partially hydrolysed by anion, their solutions have alkaline reaction.
The sulfite and bisulfite anions are both moderately strong reducing agents.
· Н2SO3 and its salts are gradually oxidized even by air oxygen in aqueous solutions:
2Na2SO3 + O2 = 2Na2SO4
· S trong oxidants oxidize SO32- practically instantly:
Н2S+4O3 + Hal20 + Н2О = Н2S+6O4 + 2НHal1- (Hal2 = Cl2, Br2, I2)
5Na2SO3 + 2KMnO4 + 3H2SO4= 5Na2SO4 + 2MnSO4 + K2SO4 + 3H2O
Na2SO3 + H2O2 = Na2SO4 + H2O
Н2SO3 demonstrates oxidizing properties at the attack of strong reductants:
Н2SO3 + 2H2S = 3S + 3H2O
Being heated, sulfites disproportionate:
4Nа2S+4O3 = Na2S-2 + 3Na2S+6O4.
POLYSULFURUOUS ACIDS. Zinc dithionite is formed at SO2 reduction with zinc dust in water:
Zn + 2SO2 = ZnS2O4.
Dithionate is also obtained by the reaction:
2NаHSO3 + Zn + H2SO3 = Na2S2O4 + ZnSO3 + 2H2O.
Dithionites are salts of dibasic dithionic acid, Н2S2O4. It is unstable and simultaneously strong acid (K1 = 5∙10-1, K2 = 4∙10-3) in aqueous solutions. It has a symmetric structure and forms tautomers:
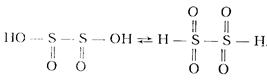
Н2S2O4 is one of the strongest reductants. In alkaline medium, it reduces even perchlorates. Salts are quickly converted into hydrogen sulfites in the presence of air oxygen:
2Na2S2O4 + O2 + 2H2O = 4NаHSO3.
Hydrogen sulfites lose water and transform into disulfite (pyrosulfite) which are salts of the disulfurous (pyrosulfurous) acid Н2S2О5 at moderate heating, for example, during crystallisation from solutions, unknown in a free state:
2NaHSO3 = Na2S2О5 + Н2O.
A more convenient method to prepare disulfites is presented below:
2SO2 + 2NaHCO3 = Na2S2О5 + 2CO2 + H2O.
This acid has the following structure:
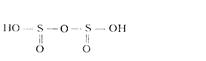
Dissolution of disulfites in water results in reverse reaction. Na2S2О5 salt is a conservator of moist corn and vine.
Oxygen - element(IV) compounds. EO2 oxides can be prepared from simple substances at combustion in air or oxygen atmosphere:
E + O2 = EO2.
Unlike SO2, EO2 oxides are polymer solids at STP: SeO2 (tsubl. = 315 °), TeO2 (m.p. = 733 °), PoO2 (m.p. = 885 °). Actually, SeO2 has a chain structure:
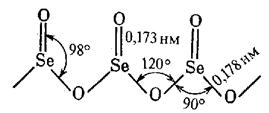
This is a tetrahedron where Se is in the state of sp3- hybridisation.
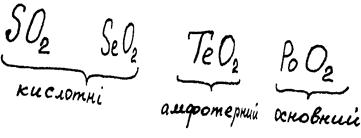
|
In the series SeO2—TeO2—PoO2 acid properties are weakened and basic properties are strengthened. The reason for this phenomenon is the growth of metallic properties of elements and also increase of ionic mechanism contribution in E—O bond. Certainly, SeO2, like SO2, is dissolved in water and alkalis easily with the formation of selenous acid, H2SeO3, which is similar to sulfurous acid:
SeO2 +H2O 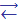 H2SeO3
H2SeO3
SeO2 + 2NaON = Na2SeO3 +H2O
TeO2 already shows amphoteric properties. It is not soluble in water, but like SeO2, is soluble in alkali solutions, forming a colourless telluride:
TeO2 + 2NaON = Na2TeO3 +H2O (TeO2 has acidic properties),
and in solutions of strong acids to form salts:
TeO2 + HI = TeI4 + 2H2O (TeO2 has basic behaviour),
TeI4 + 2HI 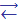 H2[TeI6].
H2[TeI6].
PoO2 is a basic oxide. That is why PoO2 has no interaction with solutions of alkali (the reaction takes place only with solid alkalis in the molten state), although gives salts with acids:
PoO2 + 2H2SO4 = Po(SO4) 2 + 2H2O.
Due to the action of strong acids on selenites and tellurites selenous and tellurious acids are formed. Unlike H2SO3, they can be obtained in the free state. H2SeO3 is a white hygroscopic solid, which easily loses water:
H2SeO3 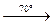 SeO2 + H2O
SeO2 + H2O
H2TeO3 has the ability to form polymers (its composition is TeO2∙ h H2O). Low soluble TeO2∙ h H2O can be deposited from concentrated solutions.
H2SeO3 and H2TeO3 are easily obtained during elemental Se and Te oxidation by concentrated HNO3.
In the series of acids H2TeO3 — H2SeO3 — H2SO3 the acid strength increases (K1 = 3.10-6, 2.10-3, 2.10-2). First of all, this behaviour is defined by the growth of chalcogen’s EN in this direction that leads to increased displacement of electrons in OH-groups to the oxygen atom and increases its polarity:
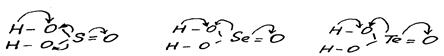
The stronger polarisation of H—O bond facilitates dissociation of acids under the influence of polar water molecules.
Elements display intermediate oxidation state in compounds Se4+ and Te+4, and these substances can be reductants and also oxidants in redox reactions, but unlike S4+ derivatives, oxidising properties are more typical of them, as evidenced by comparing Eo values:
H2SO3 + 4H+ + 4e- = S + 3H2O; E ° = 0.45 V
H2SeO3 + 4H+ + 4e- = Se + 3H2O; E ° = 0.74 V (a consequence of secondary periodicity)
H2TeO3 + 4H+ + 4e- = Te + 3H2O; E ° = 0.59 V
Therefore,
H2SO3 + HI ® no reaction
H2SeO3 + 4HI = 2I2 + Se + 3H2O
but as mentioned above: TeO2 + 4HI = TeI4 + 2H2O
Selenous acid oxidises SO2:
H2SeO3 + 2SO2 + H2O = Se + 2H2SO4
These acids can also have reducing properties (with very strong oxidants):
if H2SO3 + Br2 + H2O = H2SO4 + 2HBr
then
5H2SeO3 + 2HClO3 = 5H2SeO4 + Cl2 + H2O
Na2SeO3 + Cl2 + NaOH = Na2SeO4 + NaCl + H2O
5H2SeO3 + 2KMnO4 + 3H2SO4 = 5H2SeO4 + 2MnSO4 + K2SO4 + 3H2O
and
TeO2 + H2O2 + 2H2O = H6TeO6 ortho-telluric acid
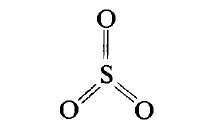
Sulfur (VI) oxide
 Production in industry. Catalytic oxidation of SO2 by air oxygen takes place in the presence of catalyst, V2O5 (440 oC):
Production in industry. Catalytic oxidation of SO2 by air oxygen takes place in the presence of catalyst, V2O5 (440 oC):
2SO2 + O2  2SO3; DН0298 =-184.4 kJ/mol
2SO3; DН0298 =-184.4 kJ/mol
In the laboratory it is commonly prepared by the reaction between sulfur dioxide and oxygen in the presence of platinum catalyst and high temperature:
2SO2 + O20  2SO3
2SO3
Initially, sulfur trioxide was prepared by heating iron(III) sulfate:
Fe2(SO4)3 = Fe2O3 + 3SO3
Itis also obtained by the dehydration of concentrated sulfuric acid with phosphorus(V) oxide:
2H2SO4 + P4O10 = 4HPO3 + 2SO3
Structure. SO3 is a nonpolar molecule that has a plane structure with the valence angle 120o (sp2- hybridisation of valence orbitals of sulfur) and equivalent bonds due to delocalised fourcentred p-bond (like SO2):

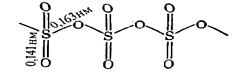
Polymorphic modifications of SO3. Molecules SO3 exist only in the gaseous phase. In the solid state, it is a polymer with (SO3)n chains:
|
This solid polymorph is referred to as a-SO3. It looks like transparent mass similar to ice; m.p. = 16.9 oC.
b - SO3. It forms when a- SO3 absorbs water. This polymer has lower molar mass than a-SO3. It does not melt, but sublime at 50oC. Externally, it is similar to asbestos.
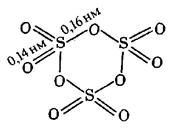
g - SO3 is the product of condensation of SO3 vapours consisting of cyclic trimer (SO3)3 where S has sp3 -hybridisation state:
|
Properties. This acid oxide reacts with basic oxides, and hydroxides. Moreover, what is most important: SO3 is an anhydride of sulfuric acid. It reacts with water rapidly:
SO3 + Н2O = Н2SO4; DН0298 = -89.2 kJ/mol
Sulfuric acid
Sulfuric acid is probably the most important chemical substance which is not found in Nature. The total world consumption is about 25 000 000 tons per year.
Production in industry:
1. Contact method. Catalytic oxidation of SO2 by air oxygen (440 oC, in the presence of V2O5 catalyst):
2SO2 + O2 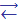 2SO3; DН0298 =-184.4 kJ/mol
2SO3; DН0298 =-184.4 kJ/mol
SO3 is absorbed by concentrated H2SO4. Usage of water is not efficient because gaseous SO3 reacts first of all with water steam and then predominately H2SO4. Fog is the product of this interaction. The solution of SO3 in H2SO4 has a technical name oleum that emphasizes its high viscosity (oleum - Lat. Oil).
H2SO4 dissolves SO3 in any proportions, so the composition of oleum is H2SO4·nSO3. Oleum contains several polysulfuric acids:
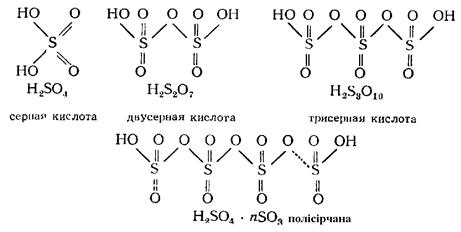
|
The content of SO3 in oleum is 20-65%. To produce acid, oleum is mixed with H2SO4 having the necessary amount of water. Under the influence of water S—O—S bonds of polysulfuric acid are destroyed and converted into H2SO4 acid of required concentration.
H2SO4 produced by this method has high purity and can be of any concentration.
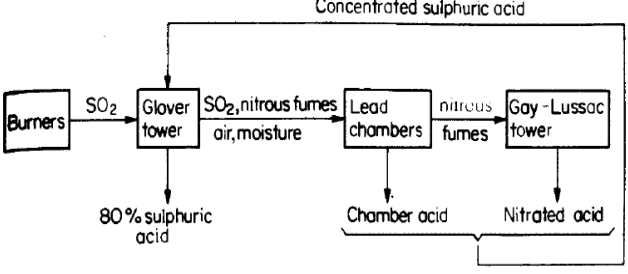
2. Lead Chamber process. It was called so because the chamber is lined with lead, which resists the action of cold sulfuric acid; the homogeneous catalyst is nitrogen oxide. The method was applied for the first time in the middle of 18th century.
It was the most important method of industrial production of H2SO4 before the modern contact method was developed. Its essence is SO2 oxidation by nitrogen oxide, NO2, in the presence of water:
SO2 + NO2 + H2O = Н2SO4 + NO
Nitrogen(II) oxide (NO) is oxidised again into the initial NO2 and returned to the reaction:
2NO + О2 = 2NO2.
The process is carried out in special towers (see scheme below). This method gives about 76% H2SO4 used in the manufacture of fertilizers:
Structure. Н2SO4 and НSO4- -ion are the distorted tetrahedrons, with sulfur atom in the centre of a polyhedron. SO42--ion has the shape of regular tetrahedron where all distances and angles are equivalent. Orbitals of sulfur have the state of sp3 -hybridisation:
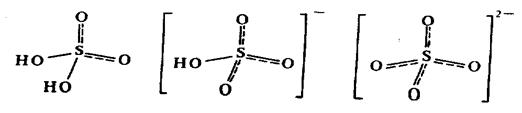
S—O bonds are very strong because of the additional p-bonding: displacement of unshared electron pairs of O atoms to the vacant 3d-orbital of S. The equal S—O bonds lengths is a consequence of delocalisation of p-electron density and the negative ion charge.
Properties. Anhydrous (100%) Н2SO4 under the normal conditions is a colourless oily liquid (m.p. = 10.4 oC). Н2SO4 molecules have intermolecular H-bonds with one another in the liquid and solid state either.
Н2SO4 azeotrope [i] contains Н2SO4 98.3 % wt. and 1.7% by weight of water, b.p. = 338.8 oC.
Н2SO4 is a strong dibasic acid in aqueous solutions:
Н2SO4 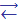 Н+ + НSO4-, K1= 1·103
Н+ + НSO4-, K1= 1·103
НSO4-  Н+ + SO42-, K2= 1,2·10-2
Н+ + SO42-, K2= 1,2·10-2
Due to the large differences in dissociation constants, Н2SO4 forms sulfates and hydrogen sulfates. The scheme of their mutual conversion is shown below:
H2SO4
K2SO4  KHSO4
KHSO4
KOH
Being dissolved Н2SO4 in water, a lot of heat liberates owing to hydrates formation. Therefore, concentrated Н2SO4 and water must be mixed with caution. To prevent spray of liquid, pour Н2SO4 (as a heavier component) into water, not vice versa. Formation of very stable hydrates explains Н2SO4 water absorption properties.
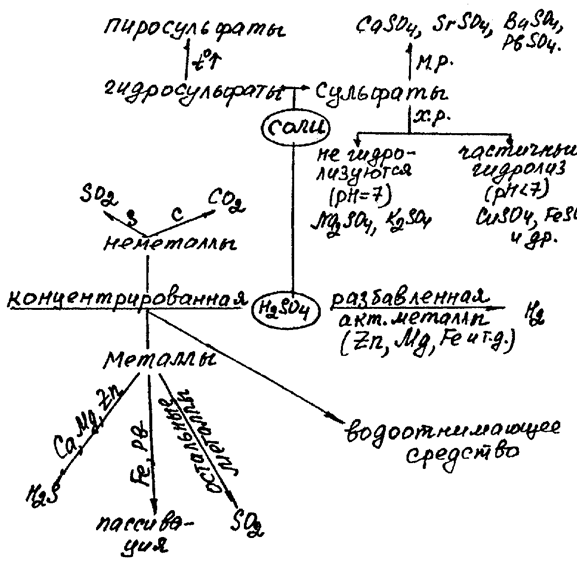
Sulfuric acid is a strong oxidising agent. Its reaction with metals depends on the concentration and activity of metal:
1. Diluted acid (oxidant is hydrogen ion H+). Therefore, it dissolves (oxidises) only metals that are situated before H in the electromotive series of metals:
Zn0 + Н2SO4(dil) = Zn+2SO4 + Н20
Zn + 2H+ = Zn2+ + H2
2. Concentrated Н2SO4 (oxidant is sulfur(VI)):
· Sulfur is reduced to SO2 at the interaction with nonactive metals (Cu, Ag, Hg):
Cu0 + 2Н2SO4(conc.) = CuSO4 + SO2 + 2H2O
· Sulfur is reduced to the elemental S or H2S (a mixture of reduction products often appears)at the interaction with active metals:
3Zn + 4Н2SO4(conc.) = 3ZnSO4 + S¯ + 4H2O
4Zn + 5Н2SO4(conc.) = 4ZnSO4 + H2S + 2H2O
It can oxidise other simple substances and compounds:
2HBr + Н2SO4(conc.) = Br2 + SO2 + 2H2O
8HI + Н2SO4(conc.) = 4I2 + H2S + 4H2O
C + 2Н2SO4(conc.) = CO2 + 2SO2 + 2H2O
S + 2Н2SO4(conc.) = 3SO2 + 2H2O
Н2SO4 is a very acidic solvent. CH3COOH and HNO3 behave as bases in Н2SO4. HClO4 is one of the strongest acids, but it is a weak one in Н2SO4 medium:
HClO4 + Н2SO4 = Н3SO4+ + ClO4-
The only acid that is stronger than Н2SO4 in its medium is hydrogen tetra(hydrogen sulfato) borate, H[B(HSO4)4]. It has not been obtained in the free state, but can be isolated from its solutions in Н2SO4:
B(OH)3 + 6Н2SO4 = [B(HSO4)4]- + 3H3O+ + 2HSO4-

Н2SO4 salts. Sulfates are very abundant. Most of them are well-soluble in water. The low soluble sulfates are CaSO4, SrSO4, BaSO4, PbSO4.
Solid hydrogen sulfates are obtained only for the most active metals (NaHSO4, KHSO4 etc.). Disulfates (pyrosulfates) are salts of disulfuric (pyrosulfuric) acid obtained at heating of hydrogen sulfates:
2NaНSO4 = Na2S2O7 + Н2O,
in turn, when heated stronger, the following reaction proceeds:
Na2S2O7 = Na2SO4+ SO3
Under the influence of water, disulfates are transformed again into hydrogen sulfates.
Chemical properties of sulfuric acid can be summarised in the scheme:
|
|
|
|
|
Дата добавления: 2014-01-11; Просмотров: 1958; Нарушение авторских прав?; Мы поможем в написании вашей работы!