
КАТЕГОРИИ:
Архитектура-(3434)Астрономия-(809)Биология-(7483)Биотехнологии-(1457)Военное дело-(14632)Высокие технологии-(1363)География-(913)Геология-(1438)Государство-(451)Демография-(1065)Дом-(47672)Журналистика и СМИ-(912)Изобретательство-(14524)Иностранные языки-(4268)Информатика-(17799)Искусство-(1338)История-(13644)Компьютеры-(11121)Косметика-(55)Кулинария-(373)Культура-(8427)Лингвистика-(374)Литература-(1642)Маркетинг-(23702)Математика-(16968)Машиностроение-(1700)Медицина-(12668)Менеджмент-(24684)Механика-(15423)Науковедение-(506)Образование-(11852)Охрана труда-(3308)Педагогика-(5571)Полиграфия-(1312)Политика-(7869)Право-(5454)Приборостроение-(1369)Программирование-(2801)Производство-(97182)Промышленность-(8706)Психология-(18388)Религия-(3217)Связь-(10668)Сельское хозяйство-(299)Социология-(6455)Спорт-(42831)Строительство-(4793)Торговля-(5050)Транспорт-(2929)Туризм-(1568)Физика-(3942)Философия-(17015)Финансы-(26596)Химия-(22929)Экология-(12095)Экономика-(9961)Электроника-(8441)Электротехника-(4623)Энергетика-(12629)Юриспруденция-(1492)Ядерная техника-(1748)
Halides and oxohalides of sulfur
|
|
|
|
Sulfur reacts directly with all halogens except for iodine.
Thus:
S2F2 (b.p. -380)
SF2 (-350)
SF4 (-400)
SF6 (-640, sublimates)
S2Cl2 (13.70)
SCl2 (590, decomposes)
SСl4 (-300, decomposes)
S2Br2 (540 at 0.2 mm Hg)
In the series of compounds of sulfur with halogens from fluorine to bromine stability of these compounds sharply drops. The most stable halide of fluorine is SF6, of chlorine is S2Cl2. The latter compound is decomposed by water:
2S2Cl2 + 2H2O = SO2 + 3S + 4HCl
Sulfur forms oxohalides:
SOCl2 is thionyl chloride
SO2Cl2 is sulfuryl chloride
SOCl2 is an extraordinarily active compound.
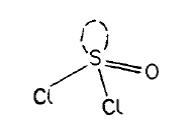
Preparation:
PCl5 + SO2 = SOCl2 + POCl3
It is hydrolysed readily:
SOCl2 + H2O = SO2 + 2HCl
SO2Cl2 is an extremely active liquid.
Preparation:
SO2 + Cl2 = SO2Cl2
CuCl2 + 2SO3 = CuSO4 + SO2Cl2
Hydrolysis of SO2Cl2 takes place:
SO2Cl2 + 2H2O = H2SO4 + 2HCl
Therefore, SO2Cl2 is a mixed anhydride or halogenanhydride.
Oxygen compounds of Se(VI), Te(VI), and Po(VI). The most important of them are SeO3 and TeO3 oxides, relevant acids and their salts.
OXIDES. EO3 are produced indirectly, whereas EO2 are the products of direct synthesis from simple substances.
SeO3 is separated during boiling of selenates with liquid SO3:
K2SeO4 + SO3 = K2SO4 + SeO3,
or at dehydration H2SeO4 by P2O5.
Molecules of SeO3 exist only in the gaseous state. Tetramers (SeO3)4 are condensed when cooling. The solid tetramer (SeO3)4 has two polymorphic modifications: vitreous and asbestos-like. It is difficult to obtain (SeO3)4 without impurities (i.e. to use recrystallisation) because it explodes while interacting with any solvent. SeO3 is a crystalline substance (m.p. = 121 °C) under normal conditions, which decomposes at t > 180 °C as follows:
2SeO3 = 2SeO2 + O2,
but sublimes in vacuum without decomposition. SeO3 is an acid oxide (acid anhydride of selenic acid):
SeO3 + H2O = H2SeO4.
In concentrated solutions, it forms a mixture of polyselenic acids similar to polysulfuric acids (H2Se2O7, m.p. = 19 °C, H2Se3O10, m.p. = 39 °C). When diluting, polyselenic acids solutions are hydrolysed to final product H2SeO4.
TeO3 is obtained by thermal dehydration of orthotelluric acid:
Н6ТeO6  ТeO3 + 3Н2O
ТeO3 + 3Н2O
TeO3 forms two polymorphs in the solid state. The reaction above leads to the a-TeO3, amorphous phase of yellow colour (density 5.1 g/cm3), which is low soluble in cold water (0.5 g /L), and is gradually transforming again into orthotelluric acid in hot water. The crystalline b- TeO3 phase of gray colour (density 6.2 g/cm3), which no longer reacts with acids and alkali solutions even when heated, can be prepared at a long-term heating of a-TeO3 in a sealed tube (300 °C).
In general, SeO3 and TeO3 oxides are soluble in alkalis forming the corresponding selenates and tellurates:
SeO3 + NaOH = Na2SeO4
SeO3 oxide’s oxidising ability is so high that oxidises HCl to Cl2 even at cooling. TeO3 oxidises HCl only when heated. Therefore, oxidising properties are weakened at the transition from SeO3 to TeO3.
ACIDS. Preparation. The highest acids of chalcogens are mostly produced by the action of strong oxidising agents:
H2Se+4O3 + H2O-12 = H2Se+6O-24 + H2O-2
Te + 3H2O2 = H6TeO6
Te + HClO3 + 3H2O = H6TeO6 + HCl.
H2SeO4 is also prepared by electrochemical oxidation of selenious acid.
H2SeO4. A convenient laboratory method of preparation.
H2SeO4 is obtained by processing Ag2SeO3 suspension with bromine water:
Ag2SeO3 + Br2 + H2O = 2AgBr¯ + H2SeO4
Selenic acid exists in the form of meta-acid, H2SeO4, telluric is the ortho-acid, H6TeO6. Both acids are colourless crystalline substances.
Structure. H2SeO4 (m.p. = 62.4 °C) can be mixed with water at any proportion; its concentrated solutions are viscous and similar to H2SO4. It has strong affinity to water and will remove 'combined' water in sugars and other organic compounds through the formation of solid hydrates H2SeO4∙H2O (m.p. = 26 °C), H2SeO4∙2H2O (m.p. = -24 °C), H2SeO4∙4H2O (m.p. = -52 °C) like H2SO4. When heated over 260 °C, selenic acid is transformed into SeO2:
2H2SeO4 = 2SeO2 + O2 + 2H2O.
The H2SeO4 molecule structure (sp3 -hybridisation) and the acid strength are similar to H2SO4 (H2SeO4, K2 = 1∙10-2; H2SO4, K2 = 1,2∙10-2; both acids have K1» 103). The significant strength is due to the presence of two very electronegative atoms of oxygen in acid residues. They shift electron density causing the growth of H—O bonds polarity and their ability to dissociate. Moreover, EO42- anions (the bond length Se—O is 0.161 nm), which appear after dissociation, are stabilised in a solution due to the negative charge uniform delocalisation between four oxygen atoms:
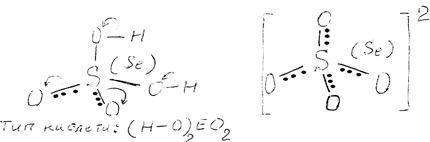
H6TeO6. Orthotelluric acid molecule has an octahedral structure (sp3d2 -hybridisation of tellurium orbitals):

Unlike H2SO4 and H2SeO4, covalent bonds Te=O are absent in H6TeO6, which would be favourable to H—O bonds polarisation and facilitate their dissociation. Therefore, H6TeO6 is a very weak acid (K1 = 2∙10-8; K2 = 9∙10-12; K3 = 3∙10-15), which is well soluble in hot water, but its solubility is limited under normal conditions (25% at 20 °C).
If heated, H6TeO6 is transformed into metatelluric acid, H2TeO4. It is much stronger than ortho- H6TeO6 form and H2TeO4 gradually turns again into orthoacid in a solution. H6TeO6 is an illustration of the well-known rule: the more a p-element atom forming an acid the more the OH-groups number associated with it, i.e. the ability of more hydrated forms formation. Tellurium (VI), in addition to other p- elements of the fifth period, has the stable coordination number 6.
When neutralising H6TeO6 by alkalis, acid salts are formed. The most abundant among them are the low soluble Na2H4TeO6 and well soluble K2H4TeO6∙3H2O. Orthotelluric acid H6TeO6 can be replaced by metals all six hydrogen atoms. For instance, Na6TeO6 neutral salt can be obtained at H6TeO6 fusion with NaOH. It gradually transforms into Na2H4TeO6∙3H2O in the humid atmosphere. There are also salts Ag6TeO6 and Hg3TeO6.
Properties. Selenic and telluric acids are powerful oxidising agents. In aqueous solutions, they are considerably stronger than H2SO4, as evidenced by E° comparison:
SeO42-+ 4H+ + 2e-= H2SeO3 + H2O; E ° = 1,15 V
H6TeO6 + 2H+ + 2e-= TeO2 + 4H2O; E ° = 1,02 V
SO42-+ 4H+ + 2e-= H2SO3 + H2O; E ° = 0,17 V
Concentrated H2SeO4 and H6TeO6 unlike H2SO4 are able to oxidise not only I‑ and Br-, but also Cl-, turning into the more stable oxidation state of these elements (+4):
2HCl + H2SeO4 = Cl2 + H2SeO3 + H2O.
Especially strong is the oxidising ability of H2SeO4. The hot anhydrous H2SeO4 dissolves well not only Ag (like H2SO4) but Au either:
2Au + 6H2SeO4 = Au2(SeO4)3 + 3SeO2 + 6H2O.
Like aqua regia (HCl + HNO3) the mixture of H2SeO4 + HCl oxidises even Pt:
Pt + 4HCl + 2H2SeO4 = PtCl4 + 2SeO2 + 4H2O
Halides. They can be obtained by direct synthesis reaction from simple substances.
| Composition, colour, state of halides of | |
| Se | Te |
| SeF6 colourless gas | TeF6 colourless gas |
| SeF4 colourless | TeF4 colourless solid |
| SeCl4 colourless solid | TeCl4 colourless solid |
| SeCl2 liquid brown | TeCl2 solid green |
| SeBr4 solid yellow | TeBr4 solid orange |
| Se2Br2 liquid red | TeBr2 solid brown |
| TeI4 gray-black |
Molecules SeF6 (m.p. = -46.6 °C) and TeF6 (m.p. = -38.6 °), like SF6, have octahedral structure, which shows sp3d2- hybridisation (Se—F and Te—F bond lengths are 0.170 nm and 0.184 nm, respectively). The most abundant are SeX4 and TeX4. By its nature, selenium halides are close to the corresponding derivatives of sulfur. For instance, Se2Cl2 and Se2Br2 are decomposed even at careful heating:
2Se2Cl2 = 3Se + Se2Cl4
Tellurium halides are significantly different from those of sulfur compounds. They have salt-like behaviour. Unlike hexafluorides of S and Se, TeF6 hydrolyses by water easily:
TeF6 + 6H2O = H6TeO6 + 6HF (it is a halogenanhydride).
Thus, in the series S—Se—Te—Po nonmetallic properties are weakened, metallic ones are increased, and ionicity in molecules grow. The chemical nature of halides changes starting with covalent (Se halides) through ionic-covalent (saltlike TeXn) to the ionic (salts PoXn).
|
|
|
|
|
Дата добавления: 2014-01-11; Просмотров: 1374; Нарушение авторских прав?; Мы поможем в написании вашей работы!